Key Points
-
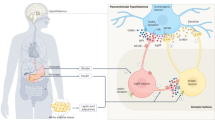
The central and peripheral melanocortin system has multifaceted roles in the control of body-weight homeostasis, sexual behaviour, and autonomic and cardiovascular functions, and so targeting this pathway has potential for the treatment of multiple clinical disorders.
-
Melanocortin peptides are derived from pro-opiomelanocortin (POMC) and act as agonists on melanocortin receptors, of which the MC3 and MC4 subtypes are primarily involved in regulations of body-weight homeostasis, sexual behaviour, autonomic and cardiovascular functions.
-
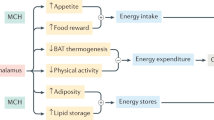
POMC-containing neurons of the arcuate (infundibular) nucleus of the hypothalamus and in the brainstem are targets for peripheral satiety signals and project to many areas of the central nervous system. Their roles in the control of complex feeding behaviour are examined.
-
Agouti-related peptide (AgRP) is a small protein factor that is part of the melanocortin system and acts as an inverse agonist on the MC3 and MC4 receptors.
-
AgRP co-exists with neuropeptide Y (NPY) in neurons originating from the arcuate nucleus and are involved in interlinked control with the central POMC neurons, and with factors such as cocaine and amphetamine regulated transcript (CART), dopamine, leptin and insulin; their dysregulations may lead to adipocity and the metabolic syndrome.
-
Mutations exist in POMC, AgRP and the MC3 and MC4 receptors that seem to have causative roles in anorexia and obesity.
-
The central melanocortin system has a key role in the regulation of male and female sexual behaviour. The role of MC3 and MC4 receptors and the relation of the central melanocortin system with the central oxytocinergic system in control of sexual behaviour is discussed.
-
Stimulation of central MC4 receptors causes activation of the sympathetic system leading to rises in blood pressure, while γ-melanocyte-stimulating hormone (γ-MSH) released from the pituitary by acting on MC3 receptors causes blood-pressure reduction by stimulation of natriuresis in the kidneys.
-
We discuss the structural and physicochemical basis for the interaction of ligands with melanocortin receptors, and give an overview of the currently available peptide and non-peptide-based compounds with activity on MC3 and MC4 receptors.
-
Of the melanocortin receptor-targeted compounds, only bremelanotide, an MC4 agonist, has entered into clinical trials. It has shown clinical benefit in Phase II trials for erectile dysfunction and female sexual dysfunction, although reports of exacerbation of high blood pressure in some subjects means that further trials are on hold.
-
There are several merits and problems of such compounds for the treatment of obesity, the metabolic syndrome, anorexia and cachexia, and sexual dysfunction; a major obstacle in the development of MC4 agonists is side effects that lead to increased blood pressure. Mixed MC3/MC4 receptor agonists could overcome this problem.
Abstract
The melanocortin system has multifaceted roles in the control of body weight homeostasis, sexual behaviour and autonomic functions, and so targeting this pathway has immense promise for drug discovery across multiple therapeutic areas. In this Review, we first outline the physiological roles of the melanocortin system, then discuss the potential of targeting melanocortin receptors by using MC3 and MC4 agonists for treating weight disorders and sexual dysfunction, and MC4 antagonists to treat anorectic and cachectic conditions. Given the complexity of the melanocortin system, we also highlight the challenges and opportunities for future drug discovery in this area.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mountjoy, K., Robbins, L., Mortrud, M. & Cone, R. The cloning of a family of genes that encode the melanocortin receptors. Science 257, 1248–1251 (1992).
Chhajlani, V. & Wikberg, J. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 309, 417–420 (1992).
Gantz, I. et al. Molecular cloning of a novel melanocortin receptor. J. Biol. Chem. 268, 8246–8250 (1993).
Gantz, I. et al. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 268, 15174–15179 (1993).
Chhajlani, V., Muceniece, R. & Wikberg, J. Molecular cloning of a novel human melanocortin receptor. Biochem. Biophys. Res. Commun. 195, 866–873 (1993).
Shutter, J. et al. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 11, 593–602 (1997).
Ollmann, M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (1997).
Miller, M. et al. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev. 7, 454–467 (1993).
Hahn, T., Breininger, J., Baskin, D. & Schwartz, M. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature Neurosci. 1, 271–272 (1998).
Douglass, J., McKinzie, A. & Couceyro, P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J. Neurosci. 15, 2471–2481 (1995).
Elias, C. et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21, 1375–1385 (1998).
Kristensen, P. et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393, 72–76 (1998).
Lambert, P. et al. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse 29, 293–298 (1998).
Menyhert, J. et al. Cocaine- and amphetamine-regulated transcript (CART) is colocalized with the orexigenic neuropeptide Y and agouti-related protein and absent from the anorexigenic α-melanocyte-stimulating hormone neurons in the infundibular nucleus of the human hypothalamus. Endocrinology 148, 4276–4281 (2007).
Cone, R. The central melanocortin system and energy homeostasis. Trends Endocrinol. Metab. 10, 211–216 (1999).
Cone, R. et al. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. 25 (Suppl. 5), 63–67 (2001).
Wikberg, J. Melanocortin receptors: new opportunities in drug discovery. Exp. Opin. Ther. Pat. 11, 61–76 (2001).
Shaw, A., Irani, B., Moore, M., Haskell-Luevano, C. & Millard, W. Ghrelin-induced food intake and growth hormone secretion are altered in melanocortin 3 and 4 receptor knockout mice. Peptides 26, 1720–1727 (2005).
Broberger, C. Brain regulation of food intake and appetite: molecules and networks. J. Intern. Med. 258, 301–327 (2005). A comprehensive review of the role of the CNS in the control of food intake and appetite.
Gropp, E. et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nature Neurosci. 8, 1289–1291 (2005).
Bouret, S., Draper, S. & Simerly, R. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304, 108–110 (2004). An interesting study showing that leptin-deficient mice do not develop normal nucleus arcuatus–hypothalamic nuclei projections of α-MSH and AgRP neurons during their postnatal second week. These defects are restored by leptin during development but not in adulthood, thus indicating prominent developmental trophic effects of leptin on food intake and appetite-controlling circuits in the brain.
Cowley, M. et al. Leptin activates anorexigenic POMC neurones through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Breen, T., Conwell, I. & Wardlaw, S. Effects of fasting, leptin, and insulin on AGRP and POMC peptide release in the hypothalamus. Brain Res. 1032, 141–148 (2005).
Watanobe, H. & Habu, S. Leptin regulates growth hormone-releasing factor, somatostatin, and α-melanocyte-stimulating hormone but not neuropeptide Y release in rat hypothalamus in vivo: relation with growth hormone secretion. J. Neurosci. 22, 6265–6271 (2002).
Guo, L., Munzberger, H., Stuart, R., Nillni, E. & Bjorbæk, C. N-acetylation of hypothalamic α-melanocyte stimulating hormone and regulation by leptin. Proc. Natl Acad. Sci. USA 10, 11797–11802 (2004).
Pinto, S. et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304, 63–64 (2004).
Zheng, H., Patterson, L., Phifer, C. & Berthoud, H. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R247–R258 (2005).
Fan, W. et al. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nature Neurosci. 7, 335–336 (2004).
Berthoud, H., Sutton, G., Townsend, R., Patterson, L. & Zheng, H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol. Behav. 89, 517–524 (2006).
Ellacott, K., Halatchev, I. & Cone, R. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology 147, 3190–3195 (2006). An elegant study indicating a pivotal role for leptin regulation of nucleus tractus solitarius POMC neurons of the brainstem.
Palkovits, M., Mezey, E. & Eskay, R. Pro-opiomelanocortin-derived peptides (ACTH/β-endrphin/α-MSH) in brainstem baroreceptor. Brain Res. 436, 323–338 (1987).
Li, G. et al. Melanocortin activation of nucleus of the solitary tract avoids anorectic tachyphylaxis and induces prolonged weight loss. Am. J. Physiol. Endocrinol. Metab. 293, E252–E258 (2007). An elegant study showing that long-term overproduction of POMC in the nucleus tractus solitarius induces long-term weight reduction and improved insulin sensitivity in rats with adult-onset obesity. This suggests a therapeutic potential for melanocortin receptor activation in this region of the CNS.
Williams, D., Bowers, R., Bartness, T., Kaplan, J. & Grill, H. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology 144, 4692–4697 (2003).
Nakamura, K., Matsumura, K., Kobayashi, S. & Kaneko, T. Sympathetic premotor neurons mediating thermoregulatory functions. Neurosci. Res. 51, 1–8 (2005).
Butler, A. et al. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nature Neurosci. 4, 605–611 (2001).
Voss-Andreae, A. et al. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148, 1550–1560 (2007).
Nogueiras, R. et al. The central melanocortin system directly controls peripheral lipid metabolism. J. Clin. Invest. 117, 3475–3488 (2007).
Zhou, Q. & Palmiter, R. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83, 1197–1209 (1995).
Lindblom, J. et al. The MC4 receptor mediates α-MSH induced release of nucleus accumbens dopamine. Neuroreport 12, 2155–2158 (2001).
Lindblom, J. et al. Chronic infusion of a melanocortin receptor agonist modulates dopamine receptor binding in the rat brain. Pharmacol. Res. 45, 119–124 (2002).
Cabeza de Vaca, S., Kim, G. & Carr, K. The melanocortin receptor agonist MTII augments the rewarding effect of amphetamine in ad-libitum-fed and food-restricted rats. Psychopharmacology (Berl.) 161, 77–85 (2002).
Hao, J., Cabeza de Vaca, S., Pan, Y. & Carr, K. Effects of central leptin infusion on the reward-potentiating effect of D-amphetamine. Brain Res. 1087, 123–133 (2006).
Polidori, C., Geary, N. & Massi, M. Effect of the melanocortin receptor stimulation or inhibition on ethanol intake in alcohol-preferring rats. Peptides 27, 144–149 (2006).
Trivedi, P. et al. Exploring the site of anorectic action of peripherally administered synthetic melanocortin peptide MT-II in rats. Brain Res. 977, 221–230 (2003).
Shaver, S., Pang, J., Wainman, D., Wall, K. & Gross, P. Morphology and function of capillary networks in subregions of the rat tuber cinereum. Cell Tissue Res. 267, 437–448 (1992).
Haynes, W., Morgan, D., Djalali, A., Sivitz, W. & Mark, A. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension 33, 542–547 (1999).
Kuo, J., Silva, A. & Hall, J. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension 41, 768–774 (2003).
Yasuda, T., Masaki, T., Kakuma, T. & Yoshimatsu, H. Hypothalamic melanocortin system regulates sympathetic nerve activity in brown adipose tissue. Exp. Biol. Med. (Maywood) 229, 235–239 (2004).
Rahmouni, K., Haynes, W., Morgan, D. & Mark, A. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J. Neurosci. 23, 5998–6004 (2003).
da Silva, A., Kuo, J. & Hall, J. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension 43, 1312–1317 (2004).
Tallam, L., Kuo, J., da Silva, A. & Hall, J. Cardiovascular, renal, and metabolic responses to chronic central administration of agouti-related peptide. Hypertension 44, 853–858 (2004).
Tallam, L., Stec, D., Willis, M., da Silva, A. & Hall, J. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension 46, 326–332 (2005).
Morse, S., Zhang, R., Thakur, V. & Reisin, E. Hypertension and the metabolic syndrome. Am. J. Med. Sci. 330, 303–310 (2005).
Humphreys, M. γ-MSH, sodium metabolism, and salt-sensitive hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R417–R430 (2004).
Zhou, A., Bloomquist, B. & Mains, R. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during pro-opiomelanocortin biosynthetic processing. J. Biol. Chem. 268, 1763–1769 (1993).
Ni, X., Pearce, D., Butler, A., Cone, R. & Humphreys, M. Genetic disruption of γ-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J. Clin. Invest. 111, 1251–1258 (2003). An important study showing that disruption of POMC processing into γ-MSH leads to salt-sensitive hypertension and that exogenously administered γ-MSH counteracts the hypertensive condition. It also shows that MC 3 receptor knockout mice also develop salt-sensitive hypertension, tentatively giving a physiological role of γ-MSH acting on MC 3 receptors in the regulation of salt balance and blood pressure
Mayan, H. et al. Dietary sodium intake modulates pituitary pro-opiomelanocortin mRNA abundance. Hypertension 28, 244–249 (1996).
Chandramohan, G., Ni, X., Kalinyak, J. & Humphreys, M. Dietary sodium modulates mRNA abundance of enzymes involved in pituitary processing of pro-opiomelanocortin. Pituitary 4, 231–237 (2001).
Ni, X.-P., Bhargava, A., Pearce, D. & Humphreys, M. Modulation by dietary intake of melanocortin 3 receptor mRNA and protein abundance in the rat kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R560–R567 (2006).
Song, C., Jackson, R., Harris, R., Richard, D. & Bartness, T. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1467–R1476 (2005).
Kawabe, T., Chitravanshi, V., Kawabe, K. & Sapru, H. Cardiovascular effects of adrenocorticotropin microinjections into the rostral ventrolateral medullary pressor area of the rat. Brain Res. 1102, 117–126 (2006).
Schwartzberg, D. & Nakane, P. ACTH-related peptide containing neurons within the medulla oblongata of the rat. Brain Res. 276, 351–356 (1983).
Mayan, H., Ni, X., Almog, S. & Humphreys, M. Suppression of γ-melanocyte-stimulating hormone secretion is accompanied by salt-sensitive hypertension in the rat. Hypertension 42, 962–967 (2003).
Saland, L. The mammalian pituitary intermediate lobe: an update on innervation and regulation. Brain Res. Bull. 6, 587–593 (2001).
Humphreys, M. Cardiovascular and renal actions of melanocyte-stimulating hormone peptides. Curr. Opin. Nephrol. Hypertens. 16, 32–38 (2007).
Ni, X. et al. Prevention of reflex natriuresis after acute unilateral nephrectomy by melanocortin receptor antagonists. Am. J. Physiol. 274, R931–R938 (1998).
Melander, O. Salt sensitivity: a consequence of the metabolic syndrome? J. Hypertens. 24, 1627–1632 (2006).
Ni, X., Butlerb, A., Cone, R. & Humphreys, M. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J. Hypertens. 24, 2239–2246 (2006).
Giuliani, D. et al. Selective melanocortin MC4 receptor agonists reverse haemorrhagic shock and prevent multiple organ damage. Br. J. Pharmacol. 150, 595–603 (2007).
Bertolini, A., Vergoni, W., Gessa, G. & Ferrari, W. Induction of sexual excitment by the action of adrenocorticotropic hormone in brain. Nature 221, 667–699 (1969).
Feder, H. & Ruf, K. Stimulation of progesterone release and estrous behaviour by ACTH in ovariectomized rodents. Endocrinology 84, 171–174 (1969).
Argiolas, A., Melis, M., Murgia, S. & Schioth, H. ACTH- and α-MSH-induced grooming, stretching, yawning and penile erection in male rats: site of action in the brain and role of melanocortin receptors. Brain Res. Bull. 51, 425–431 (2000).
Gonzalez, M., Vaziri, S. & Wilson, C. Behavioral effects of α-MSH and MCH after central administration in the female rat. Peptides 17, 171–177 (1996).
Hadley, M. Discovery that a melanocortin regulates sexual functions in male and female humans. Peptides 26, 1687–1689 (2005).
Sebhat, I. et al. Design and pharmacology of N-[(3R)-1,2,3,4-tetrahydroisoquinolinium-3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)- 2-[4-cyclohexyl-4-(1H-1,2,4-triazol-1-ylmethyl)piperidin-1-yl]-2-oxoethylamine (1), a potent, selective, melanocortin subtype-4 receptor agonist. J. Med. Chem. 45, 4589–4593 (2002).
Van der Ploeg, L. et al. A role for the melanocortin 4 receptor in sexual function. Proc. Natl Acad. Sci. USA 99, 11381–11386 (2002).
Vergoni, V., Bertolini, A., Mutulis, F., Wikberg, J. & Schioth, H. Differential influence of a selective melanocortin MC4 receptor antagonist (HS014) on melanocortin-induced behavioral effects in rats. Eur. J. Pharmacol. 362, 95–101 (1998).
Giuliano, F., Clement, P., Droupy, S., Alexandre, L. & Bernabe, J. Melanotan-II: investigation of the inducer and facilitator effects on penile erection in anaesthetized rat. Neuroscience 138, 293–301 (2006).
Wessells, H. et al. Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2 induces penile erection via brain and spinal melanocortin receptors. Neuroscience. 118, 755–762 (2003).
Caquineau, C. et al. Effects of α-melanocyte-stimulating hormone on magnocellular neurones and their activation at intromission in male rats. J. Neuroendocrinol. 18, 685–691 (2006).
Sabatier, N. et al. α-Melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J. Neurosci. 23, 10351–10358 (2003).
Bartz, J. & McInnes, L. CD38 regulates oxytocin secretion and complex social behavior. Bioessays 29, 837–841 (2007).
Pfaus, J., Shadiack, A., Van Soest, T., Tse, M. & Molinoff, P. Selective facilitation of sexual solicitation in the female rat by a melanocortin receptor agonist. Proc. Natl Acad. Sci. USA 101, 10201–10204 (2004).
Hedlund, P. PT-141 Palatin. Curr. Opin. Invest. Drugs 5, 456–462 (2003).
Meuleman, E. & Lankveld, J. Hypoactive sexual desire disorder: an underestimated condition in men. BJU Int. 95, 291–295 (2005).
Bolour, S. & Braunstein, G. Testosterone therapy in women: a review. Int. J. Impot. Res. 17, 399–408 (2005).
Enserink, M. Let's talk about sex — and drugs. Science 308, 1578 (2005).
Govaerts, C. et al. Obesity-associated mutations in the melanocortin 4 receptor provide novel insights into its function. Peptides 26, 1909–1919 (2005).
Krude, H. et al. Obesity due to pro-opiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4–10. J. Clin. Endocrinol. Metab. 88, 4633–4640 (2003).
Challis, B. et al. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Hum. Mol. Genet. 11, 1997–2004 (2002).
Vink, T. et al. Association between an agouti-related protein gene polymorphism and anorexia nervosa. Mol. Psychiatry 6, 325–328 (2001). A study showing enrichment of mutations in AgRP among patients with anorexia nervosa. This indicates that this psychiatric condition has a molecular aetiology and that it might be amenable to treatment with melanocortin receptor targeted antagonists.
Marks, D. et al. Ala67Thr polymorphism in the Agouti-related peptide gene is associated with inherited leanness in humans. Am. J. Med. Genet. A 126, 267–271 (2004).
Loos, R. et al. Two ethnic-specific polymorphisms in the human Agouti-related protein gene are associated with macronutrient intake. Am. J. Clin. Nutr. 82, 1097–1101 (2005).
Bonilla, C. et al. Agouti-related protein promoter variant associated with leanness and decreased risk for diabetes in West Africans. Int. J. Obes. 30, 715–721 (2006).
Lee, Y., Poh, L. & Loke, K. A novel melanocortin 3 receptor gene (MC3R) mutation associated with severe obesity. J. Clin. Endocrinol. Metab. 87, 1423–1426 (2002).
Tao, Y. & Segaloff, D. Functional characterization of melanocortin-3 receptor variants identify a loss-of-function mutation involving an amino acid critical for G protein-coupled receptor activation. J. Clin. Endocrinol. Metab. 89, 936–942 (2004).
Rached, M., Buronfosse, A., Begeot, M. & Penhoat, A. Inactivation and intracellular retention of the human I183N mutated melanocortin 3 receptor associated with obesity. Biochim. Biophys. Acta 1689, 229–234 (2004).
Schalin-Jantti, C. et al. Melanocortin-3-receptor gene variants in morbid obesity. Int. J. Obes. Relat. Metab. Disord. 27, 70–74 (2003).
Yiannakouris, N., Melistas, L., Kontogianni, M., Heist, K. & Mantzoros, C. The Val81 missense mutation of the melanocortin 3 receptor gene, but not the 1908c/T nucleotide polymorphism in lamin A/C gene, is associated with hyperleptinemia and hyperinsulinemia in obese Greek caucasians. J. Endocrinol. Invest. 27, 714–720 (2004).
Potoczna, N. et al. Gene variants and binge eating as predictors of comorbidity and outcome of treatment in severe obesity. J. Gastrointest. Surg. 8, 971–981 (2004).
Mackenzie, R. Obesity-associated mutations in the human melanocortin-4 receptor gene. Peptides 27, 395–403 (2006).
Hebebrand, J. et al. Binge-eating episodes are not characteristic of carriers of melanocortin-4 receptor gene mutations. Mol. Psychiatry 9, 796–800 (2004).
Tao, Y. & Segaloff, D. Functional analyses of melanocortin-4 receptor mutations identified from patients with binge eating disorder and nonobese or obese subjects. J. Clin. Endocrinol. Metab. 90, 5632–5638 (2005).
Bronner, G. et al. The 103I variant of the melanocortin 4 receptor (MC4R) is associated with low serum triglyceride levels. J. Clin. Endocrinol. Metab. 91, 535–538 (2006).
Young, E. et al. The V103I polymorphism of the MC4R gene and obesity: population based studies and meta-analysis of 29,563 individuals. Int. J. Obes. 31, 1437–1441 (2007).
Nijenhuis, W., Oosterom, J. & Adan, R. AgRP(83–132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol. Endocrinol. 15, 164–171 (2001).
Heid, I. et al. KORA Group: Association of the 103I MC4R allele with decreased body mass in 7937 participants of two population based survey. Med. Genet. 42, e21 (2005).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
Butler, A. et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141, 3518–3521 (2000).
Robbins, L. et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. 72, 827–834 (1993).
Wikberg, J. et al. New aspects on the melanocortins and their receptors. Pharmacol. Res. 42, 393–420 (2000).
Adan, R. & Kas, M. Inverse agonism gains weight. Trends Pharmacol. Sci. 24, 315–321 (2003).
Haskell-Luevano, C. et al. Three-dimensional molecular models of the hMC1R melanocortin receptor: complexes with melanotropin peptide agonists. Drug Des. Discov. 14, 197–211 (1996).
Yang, X. et al. Modeling and docking of the three-dimensional structure of the human melanocortin 4 receptor. J. Protein Chem. 22, 335–344 (2003).
Prusis, P., Frandberg, P., Muceniece, R., Kalvinsh, I. & Wikberg, J. A three dimensional model for the interaction of MSH with the melanocortin-1 receptor. Biochem. Biophys. Res. Commun. 210, 205–210 (1995).
Prusis, P. et al. Modelling of the three-dimensional structure of the human melanocortin 1 receptor, using an automated method and docking of a rigid cyclic melanocyte-stimulating hormone core peptide. J. Mol. Graph. Model. 15, 307–317 (1997).
Pogozheva, I. et al. Interactions of human melanocortin 4 receptor with nonpeptide and peptide agonists. Biochemistry 44, 11329–11341 (2005).
Lapinsh, M. et al. Proteochemometric mapping of the interaction of organic compounds with melanocortin receptor subtypes. Mol. Pharm. 67, 50–59 (2005). Introduces the use of series of multiple chimeric and wild-type MC 1 , MC 3 , MC 4 and MC 5 receptors interacting with series of diverse organic compounds for detailed proteochemometrics modelling of molecular recognition processes of melanocortin receptors.
Lapinsh, M., Prusis, P., Uhlen, S. & Wikberg, J. Improved approach for proteochemometrics modeling: application to organic compound — amine G protein-coupled receptor interactions. Bioinformatics 21, 4289–4296 (2005). Introduces a modelling approach for the concomitant proteochemometrics modelling of unlimited large series of organic compounds interacting with an unlimited large series of drug receptors.
Kubinyi, H. Chemogenomics in drug discovery. Ernst Schering Res. Found. Workshop 58, 1–19 (2006).
Sawyer, T. et al. 4-Norleucine, 7-D-phenylalanine-a-melanocyte-stimulating hormone: a highly potent α-melanotropin with ultralong biological-activity. Proc. Natl Acad. Sci. USA 77, 5754–5758 (1980).
Holder, J., Bauzo, R., Xiang, Z. & Haskell-Luevano, C. Structure–activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the mouse melanocortin receptors: part 2. Modification at the Phe position. J. Med. Chem. 45, 3073–3081 (2002).
Al-Obeidi, F., Castrucci, A., Hadley, M. & Hruby, V. Potent and prolonged-acting cyclic lactam analogs of α-melanotropin: design based on molecular dynamics. J. Med. Chem. 32, 2555–2561 (1989).
Hruby, V. et al. Cyclic lactam α-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7,Lys10] a-melanocyte-stimulating hormone-(4–10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J. Med. Chem. 38, 3454–3461 (1995).
Rosen, R., Diamond, L., Earle, D., Shadiack, A. & Molinoff, P. Evaluation of the safety, pharmacokinetics and pharmacodynamic effects of subcutaneously administered PT-141, a melanocortin receptor agonist, in healthy male subjects and in patients with an inadequate response to Viagra. Int. J. Impot. Res. 16, 135–142 (2004).
Diamond, L., Earle, D., Rosen, R., Willett, M. & Molinoff, P. Double-blind, placebo-controlled evaluation of the safety, pharmacokinetic properties and pharmacodynamic effects of intranasal PT-141, a melanocortin receptor agonist, in healthy males and patients with mild-to-moderate erectile dysfunction. Int. J. Impot. Res. 16, 51–59 (2004).
Diamond, L., Earle, D., Garcia, W. & Spana, C. Co-administration of low doses of intranasal PT-141, a melanocortin receptor agonist, and sildenafil to men with erectile dysfunction results in an enhanced erectile response. Urology 65, 755–759 (2005).
Diamond, L. et al. An effect on the subjective sexual response in premenopausal women with sexual arousal disorder by bremelanotide (PT-141), a melanocortin receptor agonist. J. Sex Med. 3, 628–638 (2006).
Palatin. Development Status: bremelanotide (formerly PT-141). Palatin web site [online], (2007).
Yan, L. et al. Potent and selective MC-4 receptor agonists based on a novel disulfide scaffold. Bioorg. Med. Chem. Lett. 15, 4611–4614 (2005).
Grieco, P., Balse, P., Weinberg, D., MacNeil, T. & Hruby, V. D-Amino acid scan of γ-melanocyte-stimulating hormone: importance of Trp8 on human MC3 receptor selectivity. J. Med. Chem. 43, 4998–5002 (2000).
Mayorov, A. et al. Development of cyclic γ-MSH analogues with selective hMC3R agonist and hMC3R/hMC5R antagonist activities. J. Med. Chem. 49, 1946–1952 (2006).
Ballet, S. et al. Novel selective human melanocortin-3 receptor ligands: use of the 4-amino-1,2,4,5-tetrahydro-2-benzazepin-3-one (Aba) scaffold. Bioorg. Med. Chem. Lett. 17, 2492–2498 (2007).
Cepoi, D. et al. Assessment of a small molecule melanocortin-4 receptor-specific agonist on energy homeostasis. Brain Res. 1000, 64–71 (2004).
Nordheim, U., Nicholson, J., Dokladny, K., Dunant, P. & Hofbauer, K. Cardiovascular responses to melanocortin 4-receptor stimulation in conscious unrestrained normotensive rats. Peptides 27, 438–443 (2006).
Tucci, F. et al. Potent and orally active non-peptide antagonists of the human melanocortin-4 receptor based on a series of trans-2-disubstituted cyclohexylpiperazines. Bioorg. Med. Chem. Lett. 15, 4389–4395 (2005).
Pontillo, J. et al. A potent and selective nonpeptide antagonist of the melanocortin-4 receptor induces food intake in satiated mice. Bioorg. Med. Chem. Lett. 15, 2541–2546 (2005).
Ye, Z. et al. Discovery and activity of (1R, 4S, 6R)-N-[(1R)-2-[4-cyclohexyl-4-[[(1,1-dimethylethyl)amino]carbonyl]-1-piperidinyl]-1-[(4-fluorophenyl)methyl]-2-oxoethyl]-2-methyl-2-azabicyclo[2.2.2]octane-6-carboxamide (3, RY764), a potent and selective melanocortin subtype-4 receptor agonist. Bioorg. Med. Chem. Lett. 15, 3501–3505 (2005).
Pontillo, J. et al. Optimization of piperazinebenzylamines with a N-(1-methoxy-2-propyl) side chain as potent and selective antagonists of the human melanocortin-4 receptor. Bioorg. Med. Chem. Lett. 15, 4615–4618 (2005).
Chen, C. W. et al. Propionylpiperazines as human melanocortin-4 receptor ligands. Bioorg. Med. Chem. Lett. 16, 4800–4803 (2006).
Doss, G. et al. Metabolic activation of a 1,3-disubstituted piperazine derivative: evidence for a novel ring contraction to an imidazoline. Chem. Res. Toxicol. 18, 271–276 (2005).
Ujjainwalla, F. et al. Design and syntheses of melanocortin subtype-4 receptor agonists: evolution of the pyridazinone archetype. Bioorg. Med. Chem. Lett. 13, 4431–4435 (2003).
Vos, T., Patane, M., Solomon, M., Blackburn, C. & Danca, M. Preparation of aroylguanidines as melanocortin MC4 receptor antagonists. WO 2004050610 A2 (2004).
Vos, T. et al. Identification of 2-{2-[2-(5-bromo-2-methoxyphenyl)-ethyl]-3-fluorophenyl}-4,5-dihydro-1H-imidazole (ML00253764), a small molecule melanocortin 4 receptor antagonist that effectively reduces tumor-induced weight loss in a mouse model. J. Med. Chem. 47, 1602–1604 (2004).
Vos, T. et al. Identification and structure–activity relationships of a new series of melanocortin-4 receptor antagonists. Bioorg. Med. Chem. Lett. 16, 2302–2305 (2006).
Poitout, L. et al. Identification of a novel series of benzimidazoles as potent and selective antagonists of the human melanocortin-4 receptor. Bioorg. Med. Chem. Lett. 17, 4464–4470 (2007).
Conde-Frieboes, K. et al. Preparation of piperazinedione derivatives for use in treating obesity. WO 2004048345 A2 (2004).
Sharma, S., Shi, Y.-Q., Wu, Z. & Rajpurohit, R. Bicyclic melanocortin-specific compounds. US Patent 2004/0152134 A1 (2004).
Tian, X. et al. Design, synthesis, and evaluation of proline based melanocortin receptor ligands. Bioorg. Med. Chem. Lett. 15, 2819–2823 (2005).
Tian, X. et al. Design and synthesis of potent and selective 1,3,4-trisubstituted-2-oxopiperazine based melanocortin-4 receptor agonists. Bioorg. Med. Chem. Lett. 16, 4668–4673 (2006).
Tian, X. et al. Design, synthesis, and evaluation of proline and pyrrolidine based melanocortin receptor agonists. A conformationally restricted dipeptide mimic approach. J. Med. Chem. 49, 4745–4761 (2006).
Tian, X. et al. Synthesis of Tic-D-Phe Ψ[CH2–CH2] isostere and its use in the development of melanocortin receptor agonists. Bioorg. Med. Chem. Lett. 16, 1721–1725 (2006).
Cain, J. et al. Design, synthesis, and biological evaluation of a new class of small molecule peptide mimetics targeting the melanocortin receptors. Bioorg. Med. Chem. Lett. 16, 5462–5467 (2006).
Nozawa, D. et al. Novel piperazines: potent melanocortin-4 receptor antagonists with anxiolytic-like activity. Bioorg. Med. Chem. Lett. 15, 2375–2385 (2007).
Benomar, Y., Roy, A., Aubourg, A., Djiane, J. & Taouis, M. Cross down-regulation of leptin and insulin receptor expression and signalling in a human neuronal cell line. Biochem. J. 388, 929–939 (2005).
Mantzoros, C. & Flier, J. Leptin as a therapeutic agent — trials and tribulations. J. Clin. Endocrinol. Metab. 85, 4000–4002 (2000).
Proietto, J. & Thorburn, A. The therapeutic potential of leptin. Expert Opin. Investig. Drugs 12, 373–378 (2003).
Franks, P. et al. Leptin predicts a worsening of the features of the metabolic syndrome independently of obesity. Obes. Res. 13, 1476–1484 (2005).
Zhang, Y., Matheny, M., Tumer, N. & Scarpace, P. Aged-obese rats exhibit robust responses to a melanocortin agonist and antagonist despite leptin resistance. Neurobiol. Aging 25, 1349–1360 (2004).
da Silva, A., Kuo, J., Tallam, L., Liu, J. & Hall, J. Does obesity induce resistance to the long-term cardiovascular and metabolic actions of melanocortin 3/4 receptor activation? Hypertension 47, 259–264 (2006).
Lu, H., Buison, A., Jen, K. & Dunbar, J. Leptin resistance in obesity is characterized by decreased sensitivity to pro-opiomelanocortin products. Peptides 21, 1479–1485 (2000).
Kuo, J., da Silva, A., Tallam, L. & Hall, J. Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension 43, 370–375 (2004).
Hallschmid, M., Smolnik, R., McGregor, G., Born, J. & Fehm, H. Overweight humans are resistant to the weight reducing effects of melanocortin 4–10. J. Clin. Endocrinol. Metab. 91, 522–525 (2006).
Sharma, S. D. et al. Melanocortin receptor-specific compounds. US Patent 20040224957.
Kang, L. et al. A selective small molecule agonist of the melanocortin-1 receptor inhibits lipopolysaccharide-induced cytokine accumulation and leukocyte infiltration in mice. J. Leukoc. Biol. 80, 897–904 (2006).
Getting, S., Lam, C., Chen, A., Grieco, P. & Perretti, M. Melanocortin 3 receptors control crystal-induced inflammation. FASEB J. 20, 2234–2241 (2006).
Getting, S., Di Filippo, C., D'Amico, M. & Perretti, M. The melanocortin peptide HP228 displays protective effects in acute models of inflammation and organ damage. Eur. J. Pharmacol. 532, 138–144 (2006).
Sharma, H., Skottner, A., Lundstedt, T., Flardh, M. & Wiklund, L. Neuroprotective effects of melanocortins in experimental spinal cord injury. An experimental study in the rat using topical application of compounds with varying affinity to melanocortin receptors. J. Neural Transm. 113, 463–476 (2006).
Vergoni, A., Bertolini, A., Wikberg, J. & Schioth, H. Selective melanocortin MC4 receptor blockage reduces immobilization stress-induced anorexia in rats. Eur. J. Pharmacol. 369, 11–15 (1999).
Scarlett, J. & Marks, D. The use of melanocortin antagonists in cachexia of chronic disease. Expert Opin. Investig. Drugs 4, 1233–1239 (2005).
Foster, A., Chen, C., Markison, S. & Marks, D. MC4 receptor antagonists: a potential treatment for cachexia. IDrugs 8, 314–319 (2005).
Madison, L. & Marks, D. Anticatabolic properties of melanocortin-4 receptor antagonists. Curr. Opin. Clin. Nutr. Metab. Care 9, 196–200 (2006).
Joppa, M., Gogas, K., Foster, A. & Markison, S. Central infusion of the melanocortin receptor antagonist agouti-related peptide (AgRP(83–132)) prevents cachexia-related symptoms induced by radiation and colon-26 tumors in mice. Peptides 28, 636–642 (2007).
Chen, C. et al. Discovery of 1-{2-[(1S)-(3-Dimethylamino-propionyl)amino-2-methylpropyl]-4-methyl-henyl}-4-[(2R)-methyl-3-(4-chlorophenyl)-propionyl]piperazine as an orally active antagonist of the melanocortin-4 receptor for the potential treatment of cachexia. J. Med. Chem. 50, 5249–5252 (2007).
Tran, J. et al. Pyrrolidinones as orally bioavailable antagonists of the human melanocortin-4 receptor with anti-cachectic activity. Bioorg. Med. Chem. Lett. 15, 5166–5176 (2007).
Xia, Y., Skoog, V., Muceniece, R., Chhajlani, V. & Wikberg, J. Polyclonal antibodies against human melanocortin MC1 receptor: preliminary immunohistochemical localisation of melanocortin MC1 receptor to malignant melanoma cells. Eur. J. Pharmacol. 288, 277–283 (1995).
Catania, A. et al. The neuropeptide α-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides 17, 675–679 (1996).
Bhardwaj, R. et al. Evidence for the differential expression of the functional a-melanocyte-stimulating hormone receptor MC-1 on human monocytes. J. Immunol. 158, 3378–3384 (1997).
Neuman-Andersen, G. et al. MC1 receptors are constitutively expressed on leucocyte subpopulations with antigen presenting and cytotoxic functions. Clin. Exp. Immunol. 126, 441–446 (2001).
Xia, Y., Wikberg, J. & Chhajlani, V. Expression of melanocortin 1 receptors in periaqueductal gray matter. Neuroreport 6, 2193–2196 (1995).
Liem, E., Joiner, T., Tsueda, K. & Sessler, D. Increased sensitivity to thermal pain and reduced subcutaneous lidocaine efficacy in redheads. Anesthesiology 102, 509–514 (2005).
Mogil, J. et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc. Natl Acad. Sci. USA 100, 4867–4872 (2003).
Xia, Y. & Wikberg, J. Localization of ACTH receptor mRNA by in situ hybridization in mouse adrenal gland. Cell Tissue Res. 286, 63–68 (1996).
Schioth, H., Chhajlani, V., Muceniece, R., Klusa, V. & Wikberg, J. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sci. 59, 797–801 (1996).
Roselli-Rehfuss, L. et al. Identification of a receptor for γ-melanotropin and other pro-opiomelanocortin peptides in the hypothalamus and limbic system. Proc. Natl Acad. Sci. USA 90, 8856–8860 (1993).
Lindblom, J., Schhioth, H., Larsson, A., Wikberg, J. & Bergstrom, L. Autoradiographic discrimination of melanocortin receptors indicates that the MC3 subtype dominates in the medial brain. Brain Res. 810, 161–171 (1998).
Chhajlani, V. Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem. Mol. Biol. Int. 38, 73–80 (1996).
Andersen, G. et al. Quantitative measurement of the levels of melanocortin receptor subtype 1,2,3 and 5 and pro-opio-melanocortin peptide gene expression in subsets of human peripheral blood leucocytes. Scand. J. Immunol. 61, 279–284 (2005).
Lam, C. & Getting, S. Melanocortin receptor type 3 as a potential target for anti-inflammatory therapy. Curr. Drug Targets. Inflamm. Allergy 3, 311–315 (2004).
Muceniece, R. et al. The MC3 receptor binding affinity of melanocortins correlates with the nitric oxide production inhibition in mice brain inflammation model. Peptides 27, 1443–1450 (2006).
Mountjoy, K., Mortrud, M., Low, M., Simerly, R. & Cone, R. Localization of the melanocortin-4 receptor (MC4R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 8, 1298–1308 (1994).
Starowicz, K., Bilecki, W., Sieja, A., Przewlocka, B. & Przewlocki, R. Melanocortin 4 receptor is expressed in the dorsal root ganglions and down-regulated in neuropathic rats. Neurosci. Lett. 358, 79–82 (2004).
Tanabe, K., Gamo, K., Aoki, S., Wada, K. & Kiyama, H. Melanocortin receptor 4 is induced in nerve-injured motor and sensory neurons of mouse. J. Neurochem. 101, 1145–1152 (2007).
van der Kraan, M. et al. Expression of melanocortin-5 receptor in secretory epithelia supports a functional role in exocrine and endocrine glands. Endocrinology 139, 2348–2355 (1998).
Fathi, Z., Iben, L. & Parker, E. Cloning, expression, and tissue distribution of a fifth melanocortin receptor subtype. Neurochem. Res. 20, 107–113 (1995).
Haskell-Luevano, C. & Monck, E. Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regul. Pept. 99, 1–7 (2001).
Siegrist, W. et al. Interactions of α-melanotropin and agouti on B16 melanoma cells: evidence for inverse agonism of agouti. Recept. Signal. Transduct Res. 17, 75–98 (1997).
Schioth, H., Muceniece, R. & Wikberg, J. Characterisation of the melanocortin 4 receptor by radioligand binding. Pharmacol. Toxicol. 79, 161–165 (1996).
Harrold, J., Widdowson, P. & Williams, G. β-MSH: a functional ligand that regulated energy homeostasis via hypothalamic MC4-R? Peptides 24, 397–405 (2005).
Schioth, H. B., Muceniece, R., Wikberg, J. E. & Chhajlani, V. Characterization of melanocortin receptor subtypes by radioligand binding analysis. Eur. J. Pharmacol. 288, 311–317 (1995).
Mandrika, I., Petrovska, R. & Wikberg, J. Melanocortin receptors form constitutive homo- and heterodimers. Biochem. Biophys. Res. Commun. 326, 349–354 (2005).
Biebermann, H. et al. Autosomal-dominant mode of inheritance of a melanocortin-4 receptor mutation in a patient with severe early-onset obesity is due to a dominant-negative effect caused by receptor dimerization. Diabetes 52, 2984–2988 (2003).
Kopanchuk, S. et al. Kinetic evicences for tandemly arranged ligand binding sites in melanocortin 4 receptors. Neurochem. Int. 49, 533–542 (2006). A kinetics modelling study showing an intricate interlinked functional control by receptor dimers on MSH peptide and low molecular mass melanocortin receptor agonist binding to MC 4 receptors.
Mutulis, F. et al. A non-peptide radioiodinated high afinity melanocortin-4 receptor ligand. J. Labelled Comp. Radiopharm. 46, 1007–1017 (2003).
Kopanchuk, S. et al. Co-operative regulation of ligand binding to melanocortin receptor subtypes: evidence for interacting binding sites. Eur. J. Pharmacol. 512, 85–95 (2005).
Shinyama, H., Masuzaki, H., Fang, H. & Flier, J. Regulation of melanocortin-4 receptor signaling: agonist-mediated desensitization and internalization. Endocrinology. 144, 1301–1314 (2003).
Nickolls, S., Fleck, B., Hoare, S. & Maki, R. Functional selectivity of melanocortin 4 receptor peptide and nonpeptide agonists: evidence for ligand-specific conformational states. J. Pharmacol. Exp. Ther. 313, 1281–1288 (2005).
Breit, A. et al. The natural inverse agonist agouti-related protein induces arrestin-mediated endocytosis of melanocortin-3 and -4 receptors. J. Biol. Chem. 281, 37447–37456 (2006).
Reizes, O., Clegg, D., Strader, A. & Benoit, S. A role for syndecan-3 in the melanocortin regulation of energy balance. Peptides 27, 274–280 (2006).
Reizes, O. et al. Transgenic expression of syndecan-1 uncovers a physiological control of feeding behavior by syndecan-3. Cell 106, 105–116 (2001).
Tkachenko, E., Rhodes, J. & Simons, M. Syndecans: new kids on the signaling block. Circ. Res. 96, 488–500 (2005).
Creemers, J. et al. Agouti-related protein is posttranslationally cleaved by proprotein convertase 1 to generate agouti-related protein (AGRP)83–132: interaction between AGRP83–132 and melanocortin receptors cannot be influenced by syndecan-3. Endocrinology 147, 1621–1631 (2006).
Gunn, T. & Barsh, G. Mahogany/attractin: en route from phenotype to function. Trends Cardiovasc. Med. 10, 76–81 (2000).
Yeo, G. & Siddle, K. Attractin' more attention — new pieces in the obesity puzzle? Biochem. J. 376, e7–e8 (2003).
Prusis, P., Uhlen, S., Petrovska, R., Lapinsh, M. & Wikberg, J. Prediction of indirect interactions in proteins. BMC Bioinformatics 22, 167 (2006). An experimental study showing that proteochemometrics can correctly model the influence of amino acids and amino-acid stretches located both near and distant from the MSH-peptide binding site in melanocortin receptors, thus showing the validity of the proteochemometric method for modelling the complex interactions of molecular recognition.
Lapinsh, M. et al. Proteochemometric modeling reveals the interaction site for Trp9 modified α-MSH peptides in melanocortin receptors. Proteins 67, 653–660 (2007).
Jegou, S., Boutelet, I. & Vaudry, H. Melanocortin-3 receptor mRNA expression in pro-opiomelanocortin neurones of the rat arcuate nucleus. Neuroendocrinology 12, 501–505 (2000).
Smith, M. et al. Melanocortins and agouti-related protein modulate the excitability of two arcuate nucleus neuron populations by alteration of resting potassium conductances. J. Physiol. 578, 425–438 (2007).
Umegaki, K. et al. The distribution of α-melanocyte stimulating hormone (α-MSH) in the central nervous system of the rat: an immunohistochemical study — Forebrain and upper brain stem. Cell. Mol. Biol. 29, 377–386 (1983).
Mounien, L., Bizet, P., Boutelet, I., Vaudry, H. & Jegou, S. Expression of melanocortin MC3 and MC4 receptor mRNAs by neuropeptide Y neurons in the rat arcuate nucleus. Neuroendocrinology 82, 164–170 (2005).
Dhillo, W. et al. Hypothalamic interactions between neuropeptide Y, agouti-related protein, cocaine- and amphetamine-regulated transcript and α-melanocyte-stimulating hormone in vitro in male rats. Neuroendocrinology 14, 725–730 (2002).
King, P., Widdowson, P., Doods, H. & Williams, G. Regulation of neuropeptide Y release from hypothalamic slices by melanocortin-4 agonists and leptin. Peptides 21, 45–48 (2000).
Molinoff, P., Shadiack, A., Earle, D., Diamond, L. & Quon, C. PT-141: a melanocortin agonist for the treatment of sexual dysfunction. Ann. NY Acad. Sci. 994, 96–102 (2003).
Hsiung, H. et al. A novel and selective β-melanocyte-stimulating hormone-derived peptide agonist for melanocortin 4 receptor potently decreased food intake and body weight gain in diet-induced obese rats. Endocrinology 146, 5257–5266 (2005).
Gadski, R., Heiman, M., Hsiung, H., Mayer, J. & Yan, L. Melanocortin-3 receptor (MC3R) agonist peptides for the treatment of metabolic disorders. WO 2005000338 A1 (2005).
Bednarek, M. et al. Potent and selective peptide agonists of a-melanotropin action at human melanocortin receptor 4: their synthesis and biological evaluation in vitro. Biochem. Biophys. Res. Commun. 286, 641–645 (2001).
Cheung, A. et al. Preparation of human melanocortin-4 receptor agonist libraries: linear peptides X-Y-DPhe7-Arg8-Trp(or 2-Nal)9-Z-NH2 . Bioorg. Med. Chem. Lett. 15, 5504–5508 (2005).
Koikov, L., Ebetino, F., Hayes, J., Cross-Doersen, D. & Knittel, J. End-capping of the modified melanocortin tetrapeptide (p-Cl)Phe-D-Phe-Arg-Trp-NH2 as a route to hMC4R agonists. Bioorg. Med. Chem. Lett. 14, 4839–4842 (2004).
Palucki, B. et al. Discovery of (2S)-N-[(1R)-2-[4-cyclohexyl-4-[[(1,1-dimethylethyl)-amino]carbonyl]-1-piperidinyl]-1-[(4-fluorophenyl)methyl]-2-oxoethyl]-4-methyl-2-piperazinecarboxamide (MB243), a potent and selective melanocortin subtype-4 receptor agonist. Bioorg. Med. Chem. Lett. 15, 171–175 (2005).
Bakshi, R. et al. 1-Amino-1,2,3,4-tetrahydronaphthalene-2-carboxylic acid as a Tic mimetic: application in the synthesis of potent human melanocortin-4 receptor selective agonists. Bioorg. Med. Chem. Lett. 15, 3430–3433 (2005).
Bakshi, R. et al. Optimization of a privileged structure leading to potent and selective human melanocortin subtype-4 receptor ligands. Bioorg. Med. Chem. Lett. 16, 1130–1133 (2006).
Palucki, B. et al. 2-Piperazinecarboxamides as potent and selective melanocortin subtype-4 receptor agonists. Bioorg. Med. Chem. Lett. 15, 1993–1996 (2005).
Ruel, R. et al. β-Alanine dipeptides as MC4R agonists. Bioorg Med. Chem. Lett. 13, 4341–4344 (2003).
Bakshi, R., Nargund, R., Palucki, B., Park, M. & Ye, Z. Preparation of carbamoylpiperazines as melanocortin-4 receptor agonists. WO 2004024720 A1 (2004).
Komatsu, Y., Shima, K., Naka, T. & Akahoshi, F. Preparation of piperidines as melanocortin 4 receptor agonists and their pharmaceutical compositions for treatment of obesity, excessive appetite, sexual dysfunction, and infertility. JP 2006282602 A2 (2006).
Lee, K. et al. Preparation of amino acid piperidinamides as melanocortin receptor agonists. WO 2005047253 A1 (2005).
Soeberdt, M., Weyermann, P., Von Sprecher, A. & Henneboehle, M. Preparation of amides derived from substituted piperidinealkylamines as melanocortin-4 receptor antagonists. WO 2004083208 A1 (2004).
Calabrese, A., Fradet, D., Hepworth, D. & Lansdell, M. Preparation of pyrrolidinyl(carbonyl) piperidines as melanocortin receptor 4 agonists for therapeutic use. US Patent 2005/0176772 A1 (2005).
Barakat, K. et al. Preparation of piperidine derivatives as melanocortin -4 receptor agonists. WO 2006019787 A2 (2006).
Soeberdt, M., Weyermann, P. & Von Sprecher, A. Substituted N-benzyllactam derivatives as melanocortin-4 receptor agonists, and their therapeutic use. EP 1538159 A1 (2005).
Soeberdt, M., Weyermann, P. & Von Sprecher, A. A. Preparation of cyclohexyl and piperidinyl derivatives, useful as melanocortin-4 receptor modulators. EP 1460069 A1 (2004).
Sings, H. & Ujjainwalla, F. Preparation of 4-aryl-1-(pyrrolidinylcarbonyl)piperidines as melanocortin-4 receptor agonists. WO 2005009950 A2 (2005).
Bakshi, R. et al. Preparation of acylated piperazine derivatives as melanocortin-4 receptor agonists for the treatment of obesity, diabetes mellitus and sexual dysfunction, and pharmaceutical compositions thereof. WO 2004078716 (2004).
Richardson, T. et al. Synthesis and structure–activity relationships of novel arylpiperazines as potent and selective agonists of the melanocortin subtype-4 receptor. J. Med. Chem. 47, 744–755 (2004).
Fisher, M. et al. Privileged structure-based ligands for melanocortin receptors — tetrahydroquinolines, indoles, and aminotetralines. Bioorg. Med. Chem. Lett. 15, 4459–4462 (2005).
Pontillo, J. et al. Structure–activity relationships of piperazinebenzylamines as potent and selective agonists of the human melanocortin-4 receptor. Bioorg. Med. Chem. Lett. 14, 4417–4423 (2004).
Pontillo, J. et al. Piperazinebenzylamines as potent and selective antagonists of the human melanocortin-4 receptor. Bioorg. Med. Chem. Lett. 14, 5605–5609 (2004).
Tran, J. et al. Identification of agonists and antagonists of the human melanocortin-4 receptor from piperazinebenzylamines. Bioorg. Med. Chem. Lett. 15, 833–837 (2005).
Jiang, W. et al. Arylpropionylpiperazines as antagonists of the human melanocortin-4 receptor. Bioorg. Med. Chem. Lett. 16, 4674–4678 (2006).
Chen, C., Tran, J., Tucci, F., Jianf, W. & Chen, W.-C. Ligands of melanocortin receptors and compositions and methods related thereto. WO 2005042516 (2005).
Chen, C. et al. Preparation of piperazinyl carboxamide and related cyclic homologs as ligands of melanocortin receptors and compositions and methods related thereto. WO 2005040109 (2005).
Pontillo, J. et al. Structure–activity relationship studies on a series of cyclohexylpiperazines bearing a phanylacetamide as ligands of the human melanocortin-4 receptor. Bioorg. Med. Chem. Lett. 15, 5237–5240 (2005).
Mutulis, F. et al. New substituted piperazines as ligands for melanocortin receptors. Correlation to the X-ray structure of “THIQ”. J. Med. Chem. 47, 4613–4626 (2004).
Briner, K. et al. Privileged structure based ligands for melanocortin-4 receptors — aliphatic piperazine derivatives. Bioorg. Med. Chem. Lett. 16, 3449–3453 (2006).
Chen, C., Tucci, F., Tran, J., Chen, W. & White, N. A preparation of piperazine derivatives, useful as ligands of melanocortin receptors. WO 2004058735 (2004).
Tran, J. et al. Design, synthesis, and SAR studies on a series of 2-pyridinylpiperazines as potent antagonists of the melanocortin-4 receptor. Bioorg. Med. Chem. Lett. 16, 3693–3696 (2006).
Shi, Q. et al. Synthesis and structure–activity relationships of novel dipeptides and reduced dipeptides as ligands for melanocortin subtype-4 receptor. Bioorg. Med. Chem. Lett. 16, 2341–2346 (2006).
Kondo, H. et al. Spiropiperidines useful for antiobesity agents. JP 2005041839 (2005).
Guo, L. et al. Preparation of cycloalkylcarbonyl or heterocycloalkylcarbonyl-substituted spiropiperidines as melanocortin-4 receptor agonists for the treatment of conditions such as obesity. WO 2004089307 (2004).
Soeberdt, M., Weyermann, P. & Von Sprecher, A. Preparation of substituted piperidine and piperazine amino acid derivatives as melanocortin-4 receptor modulators. EP 1460073 (2004).
Chaturvedula, P., Luo, G., Vig, S., Poindexter, G. & Beno, B. Preparation of amino acid heterocyclyl amides as modulators of the melanocortin-4 receptor. US Patent 2004224901 (2004).
Lee, K. et al. Preparation of amino acid aminoheterocyclyl amides as melanocortin receptor agonists. WO 2005047251 (2005).
Boyce, R., Speake, J. & Phillips, J. Preparation of piperazinylguanidinoquinazolinones as melanocortin-4 receptor (MCR-4) agonists with reduced bioaccumulation. WO 2005051391 (2005).
Nakazato, A., Ishii, T. & Nozawa, H. Use of piperazine derivatives as MC4 receptor antagonists and therapeutic agents containing them for treatment anxiety neurosis or depression. JP 2005035983 (2005).
Ujjainwalla, F. et al. Design and syntheses of melanocortin subtype-4 receptor agonists. Part 2: discovery of the dihydropyridazinone motif. Bioorg. Med. Chem. Lett. 15, 4023–4028 (2005).
Mayfield, D. et al. A role for the Agouti-related protein promoter in obesity and type 2 diabetes. Biochem. Biophys. Res. Commun. 287, 568–573 (2001).
Bai, F. et al. Functional dimorphism of two hAgRP promoter SNPs in linkage disequilibrium. J. Med. Genet. 41, 350–353 (2004).
Lee, Y., Poh, L. & Loke, K. A novel melanocortin 3 receptor gene (MC3R) mutation associated with severe obesity. J. Clin. Endocrinol. Metab. 87, 1423–1426 (2002).
Argyropoulos, G. et al. A polymorphism in the human agouti-related protein is associated with late-onset obesity. J. Clin. Endocrinol. Metab. 87, 4198–4202 (2002).
de Rijke, C. et al. Functional analysis of the Ala67Thr polymorphism in agouti related protein associated with anorexia nervosa and leanness. Biochem. Pharmacol. 70, 308–316 (2005).
Cragnolini A., Scimonelli T., Celis M.E. & Schioth H.B. The role of melanocortin receptors in sexual behavior in female rats. Neuropeptides 34, 211–215 (2000).
Wikberg, J. L. M & Prusis, P. in Chemogenomics in Drug Discovery (eds Kubinyi, H. & Muller, G.) 289–309 (Wiley, Weinham, 2004).
Acknowledgements
We are indebted to Dr P. Prusis and Dr A. Rinken for valuable comments on the manuscript. We are also indebted to Dr M. Lapinsh for providing the draft to figure 5. Support of research reported herein was obtained from the Swedish Research Council (04X-05957).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.E.S.W. holds a grant from the Swedish Research Council for studies on melanocortin receptors; holds shares in Genetta Soft AB, a Swedish company devoted to bioinformatics software; and is author of patents related to melancocortin receptors. F.M. has no competing financial interests.
Supplementary information
Supplementary information S1 (Box)
Amino acids delineating MC4 receptor selectivity of organic amines. (PDF 418 kb)
Supplementary information S2 (Box)
Possible mechanism of ligand binding to MC4 receptor dimers. (PDF 175 kb)
Related links
Related links
DATABASES
OMIM
IUPHAR Receptor Database
Glossary
- Inverse agonist
-
An agent that binds to the same receptor binding site as an agonist for that receptor but exerts the opposite pharmacological effect. Inverse agonists reverse constitutive receptor activity, thereby decreasing signalling below basal levels.
- Leptin
-
Leptin is a 16 kDa protein hormone that has a key role in regulating energy intake and energy expenditure. It is produced by adipose tissue and interacts with leptin receptors in the central nervous system.
- Anorexia and cachexia
-
Anorexia and cachexia are common in old age and associated with many severe diseases, including anorexia nervosa, a distinct psychiatric condition with an unknown aetiology.
- Afferent neurons
-
Afferent neurons are neurons that convey information from tissues and organs into the central nervous system.
- Sympathetic neural chain
-
The paravertebral ganglionic chain is located just anterior and lateral to the spinal cord and is part of the sympathetic nervous system.
- Salt-sensitive hypertension
-
Hypertension inducible by the excessive intake of sodium.
- Natriuresis
-
Excretion of excessive amounts of sodium in the urine.
- Lordotic sexual behaviour
-
Sexual behaviour of a female mammal, such as rats or mice, consisting of a ventral arching of the spine.
- Frameshift
-
Insertions or deletions of one or more nucleotides in DNA that cause a shift in its reading frame (frameshift). A significant alteration in the gene product results.
- Haploinsufficiency
-
A state in which a diploid organism has a single functional copy of a gene (with the other copy inactivated by mutation). The single functional copy of the gene does not produce enough of a gene product to bring about a wild-type condition, leading to an abnormal or diseased state.
- Bioluminescence resonance energy transfer
-
A technology that can be used to monitor protein–protein interactions. A bioluminescent luciferase-labelled molecule is used to produce a photon emission, which excites a fluorophore-labelled molecule if the two molecules are in close proximity.
- Fluorescence resonance energy transfer
-
A technology, similar to bioluminescence resonance energy transfer, used to monitor protein–protein interactions. External light is used to excite a fluorophore-labelled molecule, which then produces a photon emission, which excites a second fluorophore-labelled molecule if the two molecules are in close proximity.
- Arrestin pathway
-
A biochemical pathway that regulates the activity of G-protein-coupled receptors (GPCRs) wherein GPCR kinases phosphorylate the C terminal tail of the receptor, which is followed by arrestin binding, leading to a desensitization of signalling.
Rights and permissions
About this article
Cite this article
Wikberg, J., Mutulis, F. Targeting melanocortin receptors: an approach to treat weight disorders and sexual dysfunction. Nat Rev Drug Discov 7, 307–323 (2008). https://doi.org/10.1038/nrd2331
Issue Date:
DOI: https://doi.org/10.1038/nrd2331
This article is cited by
-
Genetics and erectile dysfunction: leveraging early foundations for new discoveries
International Journal of Impotence Research (2022)
-
Structures of active melanocortin-4 receptor–Gs-protein complexes with NDP-α-MSH and setmelanotide
Cell Research (2021)
-
A Review of Genome Wide Association Studies for Erectile Dysfunction
Current Sexual Health Reports (2019)
-
The Melanocortin Signal System of the Hypothalamus and Its Functional State in Type 2 Diabetes Mellitus and Metabolic Syndrome
Neuroscience and Behavioral Physiology (2017)
-
Morph-specific genetic and environmental variation in innate and acquired immune response in a color polymorphic raptor
Oecologia (2015)