Key Points
-
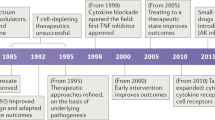
Recent progress in our understanding of signal transduction pathways that are activated during inflammation has led to new targets for anti-inflammatory drugs being explored and new drugs being developed.
-
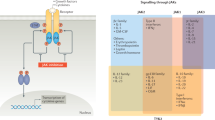
Targets have been identified in the nuclear factor-κB (NF-κB), p38 mitogen-activated protein kinase (MAPK) and Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways. Drugs that target inhibitor of NF-κB (IκB) kinase and p38 MAPK are in clinical development for rheumatoid arthritis, and a JAK3 inhibitor is being explored as an immunosuppressant.
-
Initiation of signalling by the pro-inflammatory cytokines tumour-necrosis factor, interleukin-1 (IL-1), Toll-like receptors (TLRs) and Nod-like receptors (NLRs) involves the assembly of multi-protein complexes. Targeting such complexes might yield specific agents that block inflammation.
-
TLRs and NLRs are increasingly being implicated in inflammatory diseases and targeting them selectively might have potent anti-inflammatory effects.
-
Recently, glucocorticoids have been shown to block certain genes via interference with p65–interferon regulatory factor 3 complexes activated by TLR4, with TLR3 signalling not being affected. This might prevent inflammation but leave anti-viral responses intact.
Abstract
Inflammatory diseases are a major burden on humanity, despite recent successes with biopharmaceuticals. Lack of responsiveness and resistance to these drugs, delivery problems and cost of manufacture of biopharmaceuticals mean that the search for new anti-inflammatory agents continues. Progress in our understanding of inflammatory signalling pathways has identified new targets, notably in pathways involving NF-κB, p38 MAP kinase, T lymphocyte activation and JAK/STAT. Other targets such as transcription factor complexes and components of pathways activated by TNF, Toll-like receptors and Nod-like receptors also present possibilities, and might show efficacy without being limited by effects on host defence. The challenge is to place a value on one target relative to another, and to devise strategies to modulate them.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Olsen, N. J. & Stein, C. M. New drugs for rheumatoid arthritis. N. Engl. J. Med. 350, 2167–2179 (2004).
Wang, D., Wang, M., Cheng, Y. & Fitzgerald, G. A. Cardiovascular hazard and non-steroidal anti-inflammatory drugs. Curr. Opin. Pharmacol. 5, 204–210 (2005).
Fleischmann, R. & Yocum, D. Does safety make a difference in selecting the right TNF antagonist? Arthritis Res. Ther. 6, S12–S18 (2004).
Edwards, J. C. et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 350, 2572–2581 (2004).
Kremer, J. M. et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N. Engl. J. Med. 349, 1907–1915 (2003). References 4 and 5 describe the clinical efficacy of targeting T cells in rheumatoid arthritis using anti-CD20 and CTLA4Ig.
Sandborn, W. J. et al. Natalizumab induction and maintenance therapy for Crohn's disease. N. Engl. J. Med. 353, 1912–1925 (2005).
Spina, D. The potential of PDE4 inhibitors in respiratory disease. Curr. Drug Targets Inflamm. Allergy 3, 231–236 (2004).
Chung, K. F. Phosphodiesterase inhibitors in airways disease. Eur. J. Pharmacol. 533, 110–117 (2006).
Corbin, J. D. & Francis, S. H. Cyclic GMP phosphodiesterase-5: target of sildenafil. J. Biol. Chem. 274, 13729–13732 (1999).
Herbst, R. S. & Kies, M. S. Gefitinib: current and future status in cancer therapy. Clin. Adv. Hematol. Oncol. 1, 466–472 (2003).
Perez-Soler, R. et al. Final results from a Phase II study of erlotinib monotherapy in patients with advanced non-small cell lung cancer following failure of platinum-based chemotherapy. Lung Cancer 41, S246 (2003).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353, 1659–1672 (2005).
Druker, B. J. et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukaemia and acute lymphoblastic leukaemia with the Philadelphia chromosome. N. Engl. J. Med. 344, 1038–1042 (2001). The first clinical analysis of imatinib (Gleevec) in the treatment of CML. Imatinib was found to be highly efficacious in the treatment of this disorder.
Miossec, P. The role of interleukin 1 in the pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 5, 305–308 (1987).
Brennan, F. M., Maini, R. N. & Feldmann, M. TNF-α — a pivotal role in rheumatoid arthritis? Br. J. Rheumatol. 31, 293–298 (1992).
Gay, N. J. & Keith, F. J. Drosophila Toll and IL-1 receptor. Nature 351, 355–356 (1991).
O'Neill, L. A. & Greene, C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J. Leukoc. Biol. 63, 650–657 (1998).
O'Neill, L. A. Therapeutic targeting of Toll-like receptors for inflammatory and infectious diseases. Curr. Opin. Pharmacol. 3, 3396–3403 (2003).
Sen, R. & Baltimore, D. Inducibility of κ-immunoglobulin enhancer-binding protein NF-κB by a posttranslational mechanism. Cell 47, 921–928 (1986).
Osborn, L., Kunkel, S. & Nabel, G. J. Tumor necrosis factor-α and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor κB. Proc. Natl Acad. Sci. USA 86, 2336–2340 (1989).
O'Neill, L. A. How Toll-like receptors signal: what we know and what we don't know. Curr. Opin. Immunol. 18, 3–9 (2006).
Martinon, F. & Tschopp, J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 26, 447–454 (2005).
Kolls, J. K. & Linden, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Siebenlist, U., Brown, K. & Claudio, E. Control of lymphocyte development by nuclear factor-κB. Nature Rev. Immunol. 5, 435–445 (2005).
Moynagh, P. N. The NF-κB pathway. J. Cell Sci. 118, 4589–4592 (2005).
Bonnizzi, G. et al. Activation of IKKα target genes depends on recognition of specific κB binding sites by RelB:p52 dimers. EMBO J. 23, 4202–4210 (2004).
Karin, M., Yamamoto, Y. & May Wang, Q. The IKK NF-κB system: a treasure trove for drug development. Nature Rev. Drug Discov. 3, 17–26 (2004).
di Meglio, P., Ianaro, A. & Ghosh, S. Amelioration of acute inflammation by systemic administration of a cell-permeable peptide inhibitor of NF-κB activation. Arthritis Rheum. 52, 951–958 (2005).
Luo, J. L., Kamata, H. & Karin, M. IKK/NF-κB signaling: balancing life and death — a new approach to cancer therapy. J. Clin. Invest. 115, 2625–2632 (2005).
Spano, J. P., Bay, J. O., Blay, J. Y. & Rixe, O. Proteasome inhibition: a new approach for the treatment of malignancies. Bull. Cancer 92, E61–E6, 945–952 (2005).
Proteolix, Inc. Proteolix Programs [online], (2006).
Han, J., Lee, J. D., Bibbs, L. & Ulevitch, R. J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265, 808–811 (1994).
Freshney, N. W. et al. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell 78, 1039–1049 (1994).
Lee, J. C. et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372, 739–746 (1994). Reports the identification of p38 and its targeting by pyridinyl imidazole compounds.
Kracht, M. & Saklatvala, J. Transcriptional and post-transcriptional control of gene expression in inflammation. Cytokine 20, 91–106 (2002).
Shim, J. H. et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19, 2668–2681 (2005).
Dean, J. L., Sully, G., Clark, A. R. & Saklatvala, J. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell Signal. 16, 1113–1121 (2004).
Dominguez, C., Powers, D. A. & Tamayo, N. p38 MAP kinase inhibitors: many are made, but few are chosen. Curr. Opin. Drug Discov. Devel. 8, 421–430 (2005).
Goldstein, D. M. & Gabriel, T. Pathway to the clinic: inhibition of p38 MAP kinase. A review of ten chemotypes selected for development. Curr. Top. Med. Chem. 5, 1017–1029 (2005).
Vertex, Inc. Vertex reports investigational p38 MAP kinase inhibitor, VX-702, meets primary objectives in Phase II clinical study in rheumatoid arthritis [online], (2006).
Waetzig, V. & Herdegen, T. Context-specific inhibition of JNKs: overcoming the dilemma of protection and damage. Trends Pharmacol. Sci. 26, 455–461 (2005).
Manning, A. M. & Davis, R. J. Targeting JNK for therapeutic benefit: from junk to gold. Nature Rev. Drug Discov. 2, 554–565 (2003).
Eynott, P. R., Adcock, I. M. & Chung, P. The effects of a selective c-Jun N-terminal kinase inhibition in a sensitized Brown Norway rat model of allergic asthma. Am. J. Respir. Crit. Care Med. 49, S102 (2001).
Han, Z. et al. Jun-N-terminal kinase in rheumatoid arthritis. J. Pharm. Exp. Ther. 291, 124–130 (1999).
Kassel, O. et al. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 20, 7108–7116 (2001).
Lasa, M., Abraham, S. M., Boucheron, C., Saklatvala, J. & Clark, A. R. Dexamethasone causes sustained expression of MKP-1 and phosphatase-mediated inhibition of MAPK p38. Mol. Cell. Biol. 22, 7802–7811 (2002). References 45 and 46 describe induction of MAPK phosphatase 1 by glucocorticoids, which inactivates p38 MAPK. This is likely to be an important mechanism of action through which steroids evoke anti-inflammatory responses.
O'Shea, J. J., Pesu, M., Borie, D. C. & Changelian, P. S. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nature Rev. Drug Discov. 3, 555–564 (2004).
Changelian, P. S. et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science 302, 875–878 (2003). The first report of a JAK3-specific inhibitor that has significant immunosuppressive effects.
Baslund, B. et al. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 52, 2686–2692 (2005).
Bissonnette, R. et al. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. J. Am. Acad. Dermatol. 54, 472–478 (2006).
Chiba, K., Matsuyuki, H., Maeda, Y. & Sugahara, K. Role of sphingosine 1-phosphate receptor type I in lymphocyte egress from secondary lymphoid tissues and thymus. Cell. Mol. Immunol. 3, 11–19 (2006).
Fujino, M. et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J. Pharmacol. Exp. Ther. 305, 70–77 (2003).
Medical News Today. Multiple sclerosis — FTY720, a novel once-daily oral medication, shows promising results [online], (2005).
Young, D. A. & Nickerson-Nutter, C. L. mTOR — beyond transplantation. Curr. Opin. Pharmacol. 5, 418–423 (2005).
Ghosh, P., Buchholz, M. A., Yano, S., Taub, D. & Longo, D. L. Effect of rapamycin on the cyclosporine-resistant CD28-mediated costimulatory pathway. Blood 99, 4517–4524 (2002).
Mayer, D. F. & Kushwaha, S. S. Transplant immunosuppressant agents and their role in autoimmune rheumatic diseases. Curr. Opin. Rheumatol. 15, 219–225 (2003).
Lehle, K., Birnbaum, D. E. & Preuner, J. G. Predominant inhibition of interleukin-6 synthesis in patient-specific endothelial cells by mTOR inhibitors below a concentration range where cell proliferation is affected and mitotic arrest takes place. Transplant Proc. 37, 159–161 (2005).
Dancey, J. E. Inhibitors of the mammalian target of rapamycin. Expert Opin. Investig. Drugs 14, 313–328 (2005).
Moseley, T. A., Haudenschild, D. R., Rose, L. & Reddi, A. H. Interleukin-17 and IL-17 receptors. Cytokine Growth Factor Rev. 14, 155–174 (2003).
Lubberts, E. The role of IL-17 and family members in the pathogenesis of arthritis. Curr. Opin. Investig. Drugs 4, 572–577 (2003).
Hee, M. M. et al. Small-molecule inhibition of TNF-α. Science 11, 1022–1025 (2005). Describes a small-molecule inhibitor of TNF that disrupts the TNFα trimer, thereby abolishing its biological activity.
Bouwmeester, T. et al. A physical and functional map of the human TNF/NF-κB signal transduction pathway. Nature Cell Biol. 6, 97–105 (2004). Presents a proteomic analysis of TNF signal transduction, documenting 221 molecular associations.
Janssens, S. & Beyaert, R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell 11, 293–302 (2003).
O'Neill, L. A., Fitzgerald, K. A. & Bowie, A. G. The Toll–IL-1 receptor adaptor family grows to five members. Trends Immunol. 24, 286–290 (2003).
Fitzgerald, K. A. et al. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nature Immunol. 4, 491–496 (2003).
Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 (2003).
Hacker, H. et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439, 204–207 (2006).
Oganesyan, G. Critical role of TRAF3 in the Toll-like receptor-dependent and-independent antiviral response. Nature 439, 208–211 (2006).
Honda, K. et al. Spatiotemporal regulation of MyD88–IRF-7 signalling for robust type-I interferon induction. Nature 434, 1035–1040 (2005).
Uematsu, S. et al. IRAK-1 plays an essential role for TLR7- and TLR9-mediated interferon-α induction. J. Exp. Med. 201, 915–923 (2005).
Siebl, R. et al. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am. J. Pathol. 162, 1221–1227 (2003). Describes the first indication of a role for TLR2 in rheumatoid arthritis.
Leadbetter, E. A. et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Lau, C. M. et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202, 1171–1177 (2005). References 72 and 73 implicate TLR9 and TLR7 respectively in autoantibody production.
Bartfai, T. et al. A low molecular weight mimic of Toll/IL-1 receptor/resistance domain inhibits IL-1 receptor-mediated responses. Proc. Natl Acad. Sci. USA 100, 7971–7976 (2003). Describes a peptide that can interfere with the recruitment of MyD88 to the type I IL-1R and so prevent signalling.
Harte, M. et al. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defence. J. Exp. Med. 197, 343–351 (2003).
McCoy, S. L., Kurtz, S. E., MacArthur, C. J., Trune, D. R. & Hefeneider, S. H. Identification of a peptide derived from Vaccinia virus A52R protein that inhibits cytokine secretion in response to TLR-dependent signalling and reduces in vivo bacterial-induced inflammation. J. Immunol. 174, 3006–3014 (2005).
Holtmann, M. M. RDP-58 (Sangstat medical). IDrugs 6, 1188–1194 (2003).
Travis, S. et al. RDP58 is a novel and potentially effective oral therapy for ulcerative colitis. Inflamm. Bowel Dis. 11, 713–719 (2005).
Ogura, Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411, 603–606 (2001).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603 (2001).
Strober, W., Murray, P. J., Kitani, A. & Watanabe, T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nature Rev. Immunol. 6, 9–20 (2006).
Watanabe, T., Kitani, A., Murray, P. J. & Strober, W. NOD2 is a negative regulator of TLR-2-mediated T helper Type I responses. Nature Immunol. 5, 800–808 (2004).
Maeda, S. et al. Nod2 mutation in Crohn's disease potentiates NF-κB activity and IL-1β processing. Science 307, 734–738 (2005).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Kanniganti, T. D. et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 (2006).
Siegmund, B. & Zeitz, M. Pralnacasan (Vertex pharmaceuticals). IDrugs 6, 154–158 (2003).
Agostini, L. et al. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 (2004).
Dinarello, C. A. Unravelling the NALP3/IL-1β inflammasome: a big lesson from a small mutation. Immunity 20, 243–246 (2004).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Ogawa, S. et al. Molecular determinants of crosstalk between nuclear receptors and Toll-like receptors. Cell 122, 707–721 (2005). Provides a genome-wide analysis of genes whose expression is induced by TLR3 and/or TLR4 and which are inhibited by glucocorticoids. The paper also reports that for genes induced by both TLR3 and TLR4, glucocorticoids inhibit TLR4-induced, but not TLR3-induced, gene expression. This is therefore the first demonstration suggesting that inflammation could be inhibited without limiting TLR-mediated antiviral immunity.
Wietek, C., Miggin, S. M., Jefferies, C. A. & O'Neill, L. A. Interferon regulatory factor-3-mediated activation of the interferon-sensitive response element by Toll-like receptor (TLR) 4 but not TLR3 requires the p65 subunit of NF-κ. J. Biol. Chem. 278, 50923–50931 (2003).
Humbert, M. Ciclesonide: a novel inhaled corticosteroid. Expert Opin. Investig. Drugs 13, 1349–1360 (2005).
Belvisi, M. G. et al. Preclinical profile of ciclesonide, a novel corticosteroid for the treatment of asthma. J. Pharmacol. Exp. Ther. 314, 568–574 (2005).
Lee, K. S. PPAR-γ modulates allergic inflammation through up-regulation of PTEN. FASEB J. 19, 1033–1035 (2005).
Belvisi, M. G., Hele, D. J. & Birrell, M. A. New advances and potential therapies for the treatment of asthma. BioDrugs 18, 211–223 (2004).
Staels, B. et al. Activation of human aortic smooth-muscle cells is inhibited by PPAR-α but not PPAR-γ activators. Nature 393, 790–793 (1998).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author is a co-founder of Opsona Therapeutics, which uses immune system research to develop new drugs and vaccines.
Related links
Glossary
- Biopharmaceuticals
-
Biological agents, such as antibodies or soluble receptors, that target proteins and have a therapeutic effect.
- Host defence
-
A general term for the body's system to fight infectious agents.
- Prostaglandins
-
Pro-inflammatory lipids that are formed from arachidonic acid by the action of cyclooxygenase enzymes and other downstream synthetases.
- Transcription factors
-
Intracellular proteins that bind to discrete regions of nuclear DNA and regulate gene transcription (mRNA synthesis).
- SH2 domain
-
(Src-homology-2 domain). A protein motif that recognizes and binds tyrosine-phosphorylated sequences, and thereby has a key role in relaying cascades of signal transduction.
- Cytokines
-
Proteins that are released by one cell and affect the physiology of other cells in the vicinity in a particular fashion through binding to specific receptors.
- Chemokines
-
A family of structurally related, small glycoproteins (70–90 amino acids) that have potent leukocyte activation and/or chemotactic activity. They have pivotal roles in innate and acquired immunity.
- Signalsome
-
A multi-protein complex containing the scaffold protein NEMO and the IKKs that regulates NF-κB.
- Adaptor proteins
-
Proteins that usually have several protein–protein interaction domains and increase cellular responses by recruiting other proteins to a complex.
- Combinatorial chemistry
-
The generation of large collections, or 'libraries', of compounds by synthesizing all possible combinations of a set of smaller chemical structures.
- Proteasome
-
A large protein complex that is responsible for degrading intracellular proteins that have been targeted for destruction, usually by the addition of ubiquitin polymers.
- ACR20
-
A measure developed by the American College of Rheumatology to denote a 20% improvement in outcome measures
- Phosphatidylinositol turnover
-
(PI turnover). The breakdown of phosphatidylinositol-4,5-bisphosphate to inositol-1,4,5-trisphosphate and diacylglycerol, which is mediated by phospholipase C.
- CpG motifs
-
DNA oligodeoxynucleotide sequences that include a cytosine–guanosine sequence and certain flanking nucleotides. These motifs, which are common to bacterial and viral DNA and are also incorporated in mammalian DNA, have been found to induce innate immune responses through interaction with Toll-like receptor 9.
- Caspase 1
-
A cysteine protease found in monocytes, lymphocytes, neutrophils, resting and activated T lymphocytes, placenta tissue and several B-lymphoblastoid cell lines.
- Inflammasome
-
A multi-protein complex containing Nod-like receptors, the adaptor protein ASC and caspase 1.
- Peptidomimetics
-
Small-molecule chemicals that mimic the effects of short peptides.
Rights and permissions
About this article
Cite this article
O'Neill, L. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov 5, 549–563 (2006). https://doi.org/10.1038/nrd2070
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd2070
This article is cited by
-
The acute phase reactant orosomucoid-2 directly promotes rheumatoid inflammation
Experimental & Molecular Medicine (2024)
-
Crosstalk between phytochemicals and inflammatory signaling pathways
Inflammopharmacology (2023)
-
Biomechanical signal communication in vascular smooth muscle cells
Journal of Cell Communication and Signaling (2020)
-
Mulberry leaf reduces inflammation and insulin resistance in type 2 diabetic mice by TLRs and insulin Signalling pathway
BMC Complementary and Alternative Medicine (2019)
-
Combination of NF-kB targeted siRNA and methotrexate in a hybrid nanocarrier towards the effective treatment in rheumatoid arthritis
Journal of Nanobiotechnology (2018)