Key Points
-
Despite major advances in diagnosis and treatment, such as the introduction of the statins, cardiovascular disease remains the leading cause of death in most Western countries and is rapidly emerging in the developing world.
-
Statins were initially approved on the basis of their ability to reduce levels of low-density lipoprotein (LDL) cholesterol, a biomarker of cardiovascular disease, and it was not until several years later that their ability to reduce morbidity and mortality was demonstrated.
-
The two extremes in anti-atherosclerotic drug development — use of a simple biomarker, such as LDL cholesterol, or conducting large and expensive morbidity/mortality trials — leave considerable room for intermediate endpoints.
-
Drug development teams need methods that enable the robust demonstration of anti-atherosclerotic drug efficacy via trials of a few hundred patients with relatively short follow-up before launching large morbidity/mortality trials. Atherosclerosis imaging fulfills many of these requirements, and is rapidly emerging as the most reasonable and appealing approach.
-
As discussed in this article, intravascular ultrasound (IVUS) is an imaging technology that can provide an accurate assessment of atheroma accumulation within the arterial wall. It can be used in the serial assessment of atheroma burden in response to a range of therapeutic strategies, and consequently is now a key technology in the evaluation and approval of novel drugs.
Abstract
The background use of a number of established therapies presents a key challenge for the development of novel anti-atherosclerotic agents: how to predict potential efficacy before the completion of long-term trials with endpoints such as mortality. This challenge has stimulated the search to develop intermediate measures of efficacy. Recent advances now allow intravascular ultrasound (IVUS) to provide an accurate assessment of atheroma accumulation within the arterial wall. Here we describe how IVUS can be applied to the serial assessment of atheroma burden in response to treatment with a range of anti-atherosclerotic strategies, which has resulted in its emergence as a key technology in the evaluation and approval of novel drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brown, M. S. & Goldstein, J. L. Heart attacks: gone with the century? Science 272, 629 (1996).
Libby, P. The forgotten majority: unfinished business in cardiovascular risk reduction. J. Am. Coll. Cardiol. 46, 1225–1228 (2005).
Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344, 1383–1389 (1994).
Pitt, B. et al. Pravastatin limitation of atherosclerosis in the coronary arteries (PLAC I): reduction in atherosclerosis progression and clinical events. PLAC I investigation. J. Am. Coll. Cardiol. 26, 1133–1139 (1995).
Jukema, J. W. et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS). Circulation 91, 2528–2540 (1995).
Blankenhorn, D. H. et al. Coronary angiographic changes with lovastatin therapy. The Monitored Athero-sclerosis Regression Study (MARS). The MARS Research Group. Ann. Intern. Med. 119, 969–976 (1993).
Arntz, H. R. et al. Beneficial effects of pravastatin (+/−colestyramine/niacin) initiated immediately after a coronary event (the randomized Lipid-Coronary Artery Disease [L-CAD] Study). Am. J. Cardiol. 86, 1293–1298 (2000).
Effect of simvastatin on coronary atheroma: the Multicentre Anti-Atheroma Study (MAAS). Lancet 344, 633–638 (1994).
Topol, E. J. & Nissen, S. E. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 92, 2333–2342 (1995).
Glagov, S., Weisenberg, E., Zarins, C. K., Stankunavicius, R. & Kolettis, G. J. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 316, 1371–1375 (1987).
Mintz, G. S. et al. Atherosclerosis in angiographically 'normal' coronary artery reference segments: an intravascular ultrasound study with clinical correlations. J. Am. Coll. Cardiol. 25, 1479–1485 (1995).
Schoenhagen, P., Ziada, K. M., Vince, D. G., Nissen, S. E. & Tuzcu, E. M. Arterial remodeling and coronary artery disease: the concept of 'dilated' versus 'obstructive' coronary atherosclerosis. J. Am. Coll. Cardiol. 38, 297–306 (2001).
Schoenhagen, P. et al. Association of arterial expansion (expansive remodeling) of bifurcation lesions determined by intravascular ultrasonography with unstable clinical presentation. Am. J. Cardiol. 88, 785–787 (2001).
Tuzcu, E. M. et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation 103, 2705–2710 (2001).
Nissen, S. E. et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 291, 1071–1080 (2004).
Nissen, S. E. et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 290, 2292–2300 (2003).
Nissen, S. E. et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA 292, 2217–2225 (2004).
Mintz, G. S. et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 37, 1478–1492 (2001).
Guedes, A. et al. Long-term safety of intravascular ultrasound in nontransplant, nonintervened, atherosclerotic coronary arteries. J. Am. Coll. Cardiol. 45, 559–564 (2005).
Ramasubbu, K. et al. Repeated intravascular ultrasound imaging in cardiac transplant recipients does not accelerate transplant coronary artery disease. J. Am. Coll. Cardiol. 41, 1739–1743 (2003).
Jensen, L. O., Thayssen, P., Pedersen, K. E., Stender, S. & Haghfelt, T. Regression of coronary atherosclerosis by simvastatin: a serial intravascular ultrasound study. Circulation 110, 265–270 (2004).
Okazaki, S. et al. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation 110, 1061–1068 (2004).
Nissen, S. E. et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 352, 29–38 (2005).
Cannon, C. P. et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 350, 1495–1504 (2004).
Ridker, P. M. et al. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 352, 20–28 (2005).
Nissen, S. E. et al. Very high-intensity statin therapy and coronary atherosclerosis: the ASTEROID Trial JAMA 2006 Mar 13 [epub ahead of print].
Badimon, J. J., Badimon, L. & Fuster, V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Invest. 85, 1234–1241 (1990).
Chiesa, G. et al. Recombinant apolipoprotein A-IMilano infusion into rabbit carotid artery rapidly removes lipid from fatty streaks. Circ. Res. 90, 974–980 (2002).
Nicholls, S. J. et al. Reconstituted high density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation 111, 1543–1550 (2005).
Shah, P. K. et al. High-dose recombinant apolipoprotein A-IMilano mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein E-deficient mice. Circulation 103, 3047–3050 (2001).
Brown, B. G. et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N. Engl. J. Med. 345, 1583–1592 (2001).
Taylor, A. J., Sullenberger, L. E., Lee, H. J., Lee, J. K. & Grace, K. A. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 110, 3512–3517 (2004).
Nissen, S. E. et al. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N. Engl. J. Med. 354, 1253–1263 (2006).
Dormandy, J. A. et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366, 1279–1289 (2005).
Tuzcu, E. M. et al. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. J. Am. Coll. Cardiol. 45, 1538–1542 (2005).
Eisen, H. J. et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N. Engl. J. Med. 349, 847–858 (2003).
Chambless, L. E. et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am. J. Epidemiol. 146, 483–494 (1997).
Hodis, H. N. et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann. Intern. Med. 128, 262–269 (1998).
Taylor, A. J. et al. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation 106, 2055–2060 (2002).
Greenland, P., LaBree, L., Azen, S. P., Doherty, T. M. & Detrano, R. C. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 291, 210–215 (2004).
Achenbach, S. et al. Detection of coronary artery stenoses using multi-detector CT with 16x0.75 collimation and 375 ms rotation. Eur. Heart J. 26, 1978–1986 (2005).
Salm, L. P. et al. Comprehensive assessment of patients after coronary artery bypass grafting by 16-detector-row computed tomography. Am. Heart J. 150, 775–781 (2005).
Worthley, S. G. et al. Noninvasive in vivo magnetic resonance imaging of experimental coronary artery lesions in a porcine model. Circulation 101, 2956–2961 (2000).
Fayad, Z. A. et al. In vivo magnetic resonance evaluation of atherosclerotic plaques in the human thoracic aorta: a comparison with transesophageal echocardiography. Circulation 101, 2503–2509 (2000).
Corti, R. et al. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years' follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation 106, 2884–2887 (2002).
Lima, J. A. et al. Statin-induced cholesterol lowering and plaque regression after 6 months of magnetic resonance imaging-monitored therapy. Circulation 110, 2336–2341 (2004).
Yonemura, A. et al. Effect of lipid-lowering therapy with atorvastatin on atherosclerotic aortic plaques detected by noninvasive magnetic resonance imaging. J. Am. Coll. Cardiol. 45, 733–742 (2005).
Corti, R. et al. The selective peroxisomal proliferator-activated receptor-g agonist has an additive effect on plaque regression in combination with simvastatin in experimental atherosclerosis: in vivo study by high-resolution magnetic resonance imaging. J. Am. Coll. Cardiol. 43, 464–473 (2004).
Worthley, S. G. et al. A novel nonobstructive intravascular MRI coil: in vivo imaging of experimental atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23, 346–350 (2003).
Nair, A. et al. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 106, 2200–2206 (2002).
Baldewsing, R. A. et al. Intravascular ultrasound elastography: a clinician's tool for assessing vulnerability and material composition of plaques. Stud. Health Technol. Inform. 113, 75–96 (2005).
Acknowledgements
We thank the technical expertise of the members of the Intravascular Ultrasound Core Laboratory of the Cleveland Clinic. S.J.N. is supported by a Ralph Reader Overseas Research Fellowship of the National Heart Foundation of Australia.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
S.J.N., E.M.T. and S.E.N. have received honoraria from Pfizer, Inc. E.M.T. and S.E.N. receive research support from Pfizer, Inc. I.S. receives other funding from Pfizer, Inc.
Related links
Glossary
- Biomarker
-
A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention.
- Lumen
-
The space inside the blood vessel through which blood flows.
- Glagov hypothesis
-
The proposal that in the early stages of plaque accumulation, the outer wall of the blood vessel expands or remodels, tending to preserve lumen dimensions.
- Plaques
-
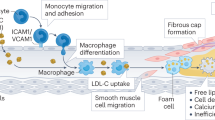
Lesions within the wall of a large artery that contain high levels of lipids, lipoproteins, foam cells, lymphocytes and smooth muscle cells. Advanced lesions are covered with a fibrous cap that can rupture and cause arterial blockage.
- Simpson's rule
-
A mathematical formula that allows for integration of area measurements to derive an estimation of volume.
- C-reactive protein
-
A peptide secreted in the setting of inflammatory states. High levels are predictive of the extent of coronary artery disease and clinical outcome.
- Foam cell
-
Macrophages in the arterial wall that ingest oxidized LDL and assume a foamy appearance. These cells secrete various substances involved in further plaque growth.
- Calcification
-
Presence of calcium within the arterial wall.
- Side-branch ostia
-
Entrance of small side branches off an artery.
- Non-uniform rotational deformity
-
(NURD). The result of rhythmical changes in rotational velocity due to frictional drag on transducer rotation. Even mild forms of NURD can result in elliptical images that decrease the accuracy of measurements.
Rights and permissions
About this article
Cite this article
Nicholls, S., Sipahi, I., Schoenhagen, P. et al. Application of intravascular ultrasound in anti-atherosclerotic drug development. Nat Rev Drug Discov 5, 485–492 (2006). https://doi.org/10.1038/nrd2040
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd2040
This article is cited by
-
Intra-coronary Imaging for the Evaluation of Plaque Modifications Induced by Drug Therapies for Secondary Prevention
Current Atherosclerosis Reports (2020)
-
Cardiovascular Outcome Trials of Diabetes and Obesity Drugs: Implications for Conditional Approval and Early Phase Clinical Development
Pharmaceutical Medicine (2017)
-
Intracoronary IVUS for Evaluation of Atherosclerosis Progression
Current Cardiovascular Imaging Reports (2012)
-
Multimodal cardiovascular magnetic resonance quantifies regional variation in vascular structure and function in patients with coronary artery disease: Relationships with coronary disease severity
Journal of Cardiovascular Magnetic Resonance (2011)
-
Vasa vasorum and molecular imaging of atherosclerotic plaques using nonlinear contrast intravascular ultrasound
Netherlands Heart Journal (2007)