Key Points
-
The greatest virtue of traditional frequentist statistical approaches may be the extreme rigour and narrowness of focus, but a side effect of this virtue is inflexibility, which in turn limits innovation in the design and analysis of clinical trials.
-
Because of this, clinical trials tend to be overly large, which increases the cost of developing new therapeutic approaches, and some patients are unnecessarily exposed to inferior experimental therapies. Bayesian approaches have the potential to address these issues.
-
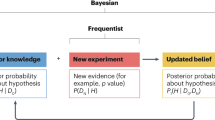
The defining characteristic of any statistical approach is how it deals with uncertainty. Unlike the frequentist approach, in the Bayesian approach, all uncertainty is measured by probability.
-
The continuous learning that is possible in the Bayesian approach enables investigators to modify trials in midcourse. Modifications include stopping the trial, adaptively assigning patients to therapies that are performing better or that will give more information about the scientific question of interest, and adding and dropping treatment arms.
-
In analysing the results of a clinical trial, the Bayesian attitude is to bring all available information to bear on the scientific question being addressed. Outside of a Bayesian perspective, such potentially important information is usually overlooked because the methodology used cannot incorporate it.
-
The Bayesian approach has several advantages in drug development, such as the process of updating knowledge gradually rather than restricting to large discrete steps measured in trials or phases. Another advantage is that it is specifically tied to decision making, within a particular trial, within a drug development programme, and within establishing a company's portfolio of drugs under development.
-
Therapeutic areas in which the clinical endpoints are observed early stand to benefit most from an increase in the use of Bayesian approaches. Similiarly, diseases such as cancer in which there is a burgeoning number of biomarkers available for assessing the disease's progress will also benefit.
Abstract
Bayesian statistical methods are being used increasingly in clinical research because the Bayesian approach is ideally suited to adapting to information that accrues during a trial, potentially allowing for smaller more informative trials and for patients to receive better treatment. Accumulating results can be assessed at any time, including continually, with the possibility of modifying the design of the trial, for example, by slowing (or stopping) or expanding accrual, imbalancing randomization to favour better-performing therapies, dropping or adding treatment arms, and changing the trial population to focus on patient subsets that are responding better to the experimental therapies. Bayesian analyses use available patient-outcome information, including biomarkers that accumulating data indicate might be related to clinical outcome. They also allow for the use of historical information and for synthesizing results of relevant trials. Here, I explain the rationale underlying Bayesian clinical trials, and discuss the potential of such trials to improve the effectiveness of drug development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Berry, D. A. Statistics: A Bayesian Perspective (Duxbury, California, 1996). Provides an elementary introduction to the Bayesian approach.
Christensen R. Testing Fisher, Neyman, Pearson, and Bayes. Am. Statistician 59, 121–126 (2005).
Berry, D. A. Interim analysis in clinical trials: the role of the likelihood principle. Am. Statistician 41, 117–122 (1987).
Berger, J. O. & Berry, D. A. Statistical analysis and the illusion of objectivity. Am. Scientist 76, 159–165 (1988).
Berger, J. O. & Wolpert, R. L. The Likelihood Principle (Institution of Mathematical Statistics, California, 1988).
Berry, D. A. & Stangl, D. K. Bayesian Biostatistics (Marcel Dekker, New York, 1996).
Buzdar, A. U. et al. Significantly higher pathological complete remission rate following neoadjuvant therapy with trastuzumab, paclitaxel and epirubicin-containing chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J. Clin. Oncol. 23, 3676–3685 (2005).
Inoue, L. Y. T., Berry, D. A. & Parmigiani, G. Relationship between Bayesian and frequentist sample size determination. Am. Statistician 59, 79–87 (2005).
Cheng, Y., Su, F. & Berry, D. A. Choosing sample size for a clinical trial using decision analysis. Biometrika 90, 923–936 (2003).
Giles, F. J. et al. Adaptive randomized study of idarubicin and cytarabine versus troxacitabine and cytarabine versus troxacitabine and idarubicin in untreated patients 50 years or older with adverse karyotype acute myeloid leukemia. J. Clin. Oncol. 21, 1722–1727 (2003).
Berry, D. A. et al. in Case Studies in Bayesian Statistics V (eds Gatsonis, C., Carlin, B. & Carriquiry, A.) 99–181 (Springer, New York, 2001). This chapter gives a detailed description of an adaptive dose-finding trial in stroke taking a Bayesian perspective. Doses are assigned based on accumulating data to maximize the efficiency of the trial. An additional innovation is allowing for proceeding seamlessly from Phase II to Phase III.
Malakoff, D. Statistics: Bayes offers a 'new' way to make sense of numbers. Science 286, 1460–1464 (1999).
Farr-Jones, S. Better statistics. BioCentury 9, 1–6 (2001).
Krams, M. et al. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN). An adaptive dose–response study of UK-279, 276 in acute ischemic stroke. Stroke 34, 2543–2548 (2003).
Berry, D. A. in Cancer Medicine 6th edn (eds Holland, J. et al.) 465–478 (Decker, London, 2003).
Berry, D. A. Bayesian statistics and the efficiency and ethics of clinical trials. Statistical Sci. 19, 175–187 (2004).
Spiegelhalter, D. J., Abrams, K. R. & Myles, J. P. Bayesian Approaches to Clinical Trials and Health-Care Evaluation (Wiley, Chichester, 2004). This is a comprehensive reference for Bayesian statistical methodology. It is an ideal guide for drug developers and medical researchers generally.
Berry, S. M. & Berry, D. A. Accounting for multiplicities in assessing drug safety: a three-level hierarchical mixed model. Biometrics 60, 418–426 (2004). This is a methodology paper that applies Bayesian borrowing in an important aspect of drug develop-ment. The methodology is useful more generally, including incorporating biological knowledge about genetic pathways in the analysis of microarray data.
Inoue, L. Y. T., Thall, P. & Berry, D. A. Seamlessly expanding a randomized phase II trial to phase III. Biometrics 58, 264–272 (2002). This paper demonstrates important advantages of the Bayesian approach in drug-trial design. It uses frequent interim analyses based on predictive probabilities and models relationships between the primary endpoint and early measures of a patient's performance.
Hennekens, C. H. et al. Additive benefits of pravastatin and aspirin to decrease risks of cardiovascular disease: Randomized and observational comparisons of secondary prevention trials and their meta-analysis. Arch. Intern. Med. 164, 40–44 (2004).
Berry, S. M. et al. Bayesian survival analysis with nonproportional hazards: Metanalysis of pravastatin-aspirin. J. Am. Statistical Assoc. 99, 36–44 (2004).
Edwards, W., Lindman, H. & Savage, L. J. Bayesian statistical inference for psychological research. Psychol. Rev. 70, 193–242 (1963).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
D.A.B. has been a paid consultant for Bristol-Myers Squibb.
Related links
Glossary
- Frequentist
-
An approach to statistical inference that is an inverse of the Bayesian approach. The focus is on the probability of results of a trial — usually including the observed data — assuming that a particular hypothesis is true. For example, a frequentist P-value is the probability of observing results as extreme as or more extreme than the observed results assuming that the null hypothesis is true.
- Bayesian
-
An approach to statistical inference that uses Bayes rule. The focus is on the probability that a hypothesis is true given the available evidence.
- Parameter
-
A population characteristic, such as the tumour-response rate when patients are treated with a particular therapy. Parameters serve to index the possible distributions of trial results. Parameters can never be known precisely because populations can never be fully assessed. Statistics are analogous to parameters but apply for samples from a population and so statistics are known when the sample becomes available. Statistics are commonly used to estimate parameters, such as when a response rate from a clinical trial is used to estimate the response rate of all patients having the disease in question.
- Null hypothesis
-
An underlying state of nature in which there is 'no difference' among two or more treatments or interventions. A hypothesis is a specific value of a parameter. So a null hypothesis is when the parameter is an indicator of treatment effect and the value specified corresponds to no effect.
- Stopping rule
-
Prespecified conditions that indicate what interim results would lead to stopping the trial. Possible decisions are to stop the trial's accrual of new patients or to announce the results of the trial. Even though a trial's accrual is complete and all patients have been treated, the trial's DSMB can keep the trial's results under wraps when so indicated by the stopping rule.
- Bayes rule
-
Mathematical theorem of inverse probabilities. Used to relate the probability of a hypothesis given experimental evidence to the probability of the experimental evidence given the hypothesis.
- Trastuzumab
-
An anti-HER2/neu antibody that is approved for the treatment of HER2-positive metastatic breast cancer.
- Interim analysis
-
An analysis of trial results that takes place before the final analysis. A trial design can include a single interim analysis, at half the planned number of events, say, or more than 10 interim analyses. Usually, the focus of an interim analysis is efficacy, with safety issues addressed throughout a trial without specifically calling them 'interim analyses.' Consequences of interim analyses are modifications of the future course of the trial, usually stopping the trial. In most trials, DSMBs oversee the interim analyses and make recommendations to the sponsor concerning whether a trial modification is appropriate.
- Data and Safety Monitoring Board
-
(DSMB). Also known as Data Monitoring Committee, this is a panel of clinicians, statisticians, community members and possibly other experts that is charged with ensuring the safety of the trial participants and also with protecting their contributions to clinical research by preserving the scientific integrity of the trial. Members are independent from all other aspects of the trial and from the trial's sponsoring agencies or companies.
- Placebo controls
-
Patients in a concurrent control group who are given a treatment that is indistinguishable from the experimental drug but which is inert — sometimes called a 'sugar pill.' Patients are 'blinded' or 'masked' as to the treatment they have been assigned. If clinicians are also masked as to the patients' assignments then the trial is 'double-blind.'
- Randomized controls
-
Concurrent controls who are designated to be controls by a randomization device instead of receiving the experimental therapy. The proportion of controls depends on the trial's design; in many two-armed trials the proportion is 50%. In randomized adaptive trials, the proportion assigned to the control group varies over the course of the trial and depends on the most recent by-arm outcome information.
- Historical controls
-
Individuals from a database or previous clinical trial(s) who received a therapy that is an appropriate comparison for an experimental therapy being investigated in a clinical trial.
- Concurrent controls
-
Participants who take part in a clinical trial but do not receive the therapy under investigation. These participants are as similar as possible to those who receive the experimental therapy. They serve as a comparison group for assessing the benefit of the experimental therapy.
- Active controls
-
Patients who receive or previously received a therapy that has been shown, or at least is perceived to be, effective for the disease or condition in question. A trial can be designed to show that a group of patients receiving an experimental therapy perform better than (superiority trial) or not worse than (non-inferiority trial) an active control.
- Hierarchical model
-
A statistical model with more than one level of (nested) experimental units. An example is patients within trials. Patients are assumed to have a distribution with a parameter depending on the trial in which they participate, and the trial parameters themselves are regarded as having been sampled from some population. Statistical inferences concern individual trial parameters and also parameters that characterize the distribution of trial parameters.
Rights and permissions
About this article
Cite this article
Berry, D. Bayesian clinical trials. Nat Rev Drug Discov 5, 27–36 (2006). https://doi.org/10.1038/nrd1927
Issue Date:
DOI: https://doi.org/10.1038/nrd1927
This article is cited by
-
Pragmatic randomized controlled trials: strengthening the concept through a robust international collaborative network: PRIME-9—Pragmatic Research and Innovation through Multinational Experimentation
Trials (2024)
-
Challenging current scientific practice: how a shift in research methodology could reduce animal use
Lab Animal (2024)
-
Using Bayesian Dynamic Borrowing to Maximize the Use of Existing Data: A Case-Study
Therapeutic Innovation & Regulatory Science (2024)
-
baymedr: an R package and web application for the calculation of Bayes factors for superiority, equivalence, and non-inferiority designs
BMC Medical Research Methodology (2023)
-
Bayesian methods: a potential path forward for sepsis trials
Critical Care (2023)