Key Points
-
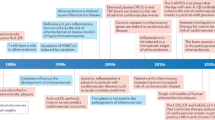
Recent evidence has shown that the statins, a class of drugs originally designed to manage cardiovascular disorders by lowering cholesterol might, in part, mediate their protective effects by reducing inflammation. Statin-mediated inhibition of inflammation might affect outcomes in cardiovascular trials independently of the extent of lipid-lowering achieved.
-
The role of inflammation in atherogenesis, combined with the observation that statins show benefit in conditions that are not strongly associated with hyperlipidaemia, led to studies into the additional effects of statins.
-
In the clinic, evidence that statins could have favorable and clinically relevant anti-inflammatory effects independent of lipid lowering is derived from studies of endothelial function, clinical trials of organ transplantation, and clinical trials of myocardial infarction and stroke prevention.
-
Of potential interest is the statin-induced reduction of C-reactive protein (CRP), a marker for inflammation; recent data suggests that the CRP-lowering effect of statins might, in addition to lipid lowering, be relevant for progression of disease.
-
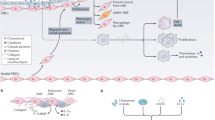
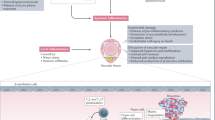
Data from experiments in cell culture and animal models show that statins can induce the cellular accumulation of endothelial nitric oxide synthase; inhibit the expression of adhesion molecules and chemokines that recruit inflammatory cells; inhibit expression of pro-coagulant factors and induce anti-coagulant substances; inhibit proliferation and promote apoptosis of vascular smooth muscle cells; and ameliorate platelet hyper-reactivity.
-
Pathways/factors implicated in the cellular effects of statins include the cholesterol biosynthesis pathway, Ras/Rho, nuclear factor-κB and activator protein-1-mediated pro-inflammatory pathways, and nuclear factors such as peroxisome proliferator-activated receptor and Kruppel-like factor-2.
-
Future studies of the benefits of statins will need to focus on anti-inflammatory targets and will need to take into account interindividual variation to the drugs.
Abstract
Chronic inflammation is a key feature of vascular disease states such as atherosclerosis. Multiple clinical studies have shown that a class of medications termed statins lower cardiovascular morbidity and mortality. Originally developed to lower serum cholesterol, increasing evidence suggests that these medications have potent anti-inflammatory effects that contribute to their beneficial effects in patients. Here, we discuss the clinical and experimental evidence underlying the anti-inflammatory effects of these agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Libby, P. Inflammation in atherosclerosis. Nature 420, 868?874 (2002).
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685?1695 (2005). References 1 and 2 are outstanding reviews on inflammation and atherogenesis.
Zawadzki, J. V. & Furchgott, R. F. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288, 373?376 (1980).
Owen, W. G. & Esmon, C. T. Functional properties of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. J. Biol. Chem. 256, 5532?5535 (1981).
Esmon, C. T. & Owen, W. G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc. Natl Acad. Sci. USA 78, 2249?2252 (1981).
Cybulsky, M. I. & Gimbrone, M. A., Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251, 788?791 (1991).
Yla-Herttuala, S. et al. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc. Natl Acad. Sci. USA 88, 5252?5256 (1991).
Wang, J. M. et al. Expression of monocyte chemotactic protein and interleukin-8 by cytokine-activated human vascular smooth muscle cells. Arterioscler. Thromb. 11, 1166?1174 (1991).
Gu, L. et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 2, 275?281 (1998).
Boring, L., Gosling, J., Cleary, M. & Charo, I. F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894?897 (1998).
Owens, G. K., Kumar, M. S. & Wamhoff, B. R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767?801 (2004).
Davies, M. J. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation 94, 2013?2020 (1996).
Virmani, R., Burke, A. P., Farb, A. & Kolodgie, F. D. Pathology of the unstable plaque. Prog. Cardiovasc. Dis. 44, 349?356 (2002).
Galis, Z. S., Sukhova, G. K., Lark, M. W. & Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Invest. 94, 2493?2503 (1994).
Sukhova, G. K. et al. Evidence for increased collagenolysis by interstitial collagenases-1 and-3 in vulnerable human atheromatous plaques. Circulation 99, 2503?2509 (1999).
Lee, R. T. & Libby, P. The unstable atheroma. Arterioscler. Thromb. Vasc. Biol. 17, 1859?1867 (1997).
Danenberg, H. D. et al. Increased thrombosis after arterial injury in human C-reactive protein-transgenic mice. Circulation 108, 512?515 (2003). This paper provides the first in vivo evidence that CRP affects vascular thrombosis.
Lindahl, B., Toss, H., Siegbahn, A., Venge, P. & Wallentin, L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N. Engl. J. Med. 343, 1139?1147 (2000).
Balbay, Y. et al. Circulating interleukin-1β, interleukin-6, tumor necrosis factor-α, and soluble ICAM-1 in patients with chronic stable angina and myocardial infarction. Angiology 52, 109?114 (2001).
Yamashita, H., Shimada, K., Seki, E., Mokuno, H. & Daida, H. Concentrations of interleukins, interferon, and C-reactive protein in stable and unstable angina pectoris. Am. J. Cardiol. 91, 133?136 (2003).
Varo, N. et al. Soluble CD40L: risk prediction after acute coronary syndromes. Circulation 108, 1049?1052 (2003).
Ridker, P. M., Cushman, M., Stampfer, M. J., Tracy, R. P. & Hennekens, C. H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 336, 973?979 (1997).
Ridker, P. M., Hennekens, C. H., Roitman-Johnson, B., Stampfer, M. J. & Allen, J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet 351, 88?92 (1998).
Ridker, P. M., Rifai, N., Stampfer, M. J. & Hennekens, C. H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101, 1767?1772 (2000).
Schonbeck, U., Varo, N., Libby, P., Buring, J. & Ridker, P. M. Soluble CD40L and cardiovascular risk in women. Circulation 104, 2266?2268 (2001).
Malik, I. et al. Soluble adhesion molecules and prediction of coronary heart disease: a prospective study and meta-analysis. Lancet 358, 971?976 (2001).
Ridker, P. M., Hennekens, C. H., Buring, J. E. & Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 342, 836?843 (2000). Clinical demonstration that the predictive value of inflammation as assessed by CRP is independent of and additive to that of cholesterol.
Ridker, P. M., Rifai, N., Cook, N. R., Bradwin, G. & Buring, J. E. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA 294, 326?333 (2005).
Paul, A. et al. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation 109, 647?655 (2004).
Hirschfield, G. M. et al. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc. Natl Acad. Sci. USA 102, 8309?8314 (2005).
Trion, A. et al. No effect of C-reactive protein on early atherosclerosis development in apolipoprotein E*3-Leiden/human c-reactive protein transgenic mice. Arterioscler. Thromb. Vasc. Biol. 25, 1635?1640 (2005).
Endo, A., Kuroda, M. & Tsujita, Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J. Antibiot. (Tokyo) 29, 1346?1348 (1976). Identification of statins as inhibitors of cholesterol synthesis.
Istvan, E. S. & Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 292, 1160?1164 (2001).
Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. Nature 343, 425?430 (1990).
Gordon, T. & Kannel, W. B. Premature mortality from coronary heart disease. The Framingham study. JAMA 215, 1617?1625 (1971).
Kannel, W. B., Castelli, W. P., Gordon, T. & McNamara, P. M. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann. Intern. Med. 74, 1?12 (1971).
Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344, 1383?1389 (1994).
Shepherd, J. et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 333, 1301?1307 (1995).
Sacks, F. M. et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 335, 1001?1009 (1996).
Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N. Engl. J. Med. 339, 1349?1357 (1998).
Downs, J. R. et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 279, 1615?1622 (1998).
Sever, P. S. et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial ? Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 361, 1149?1158 (2003).
MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360, 7?22 (2002).
Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285, 2486?2497 (2001).
Blauw, G. J., Lagaay, A. M., Smelt, A. H. & Westendorp, R. G. Stroke, statins, and cholesterol. A meta-analysis of randomized, placebo-controlled, double-blind trials with HMG-CoA reductase inhibitors. Stroke 28, 946?950 (1997).
Hebert, P. R., Gaziano, J. M., Chan, K. S. & Hennekens, C. H. Cholesterol lowering with statin drugs, risk of stroke, and total mortality. An overview of randomized trials. JAMA 278, 313?321 (1997).
Kobashigawa, J. A. et al. Effect of pravastatin on outcomes after cardiac transplantation. N. Engl. J. Med. 333, 621?627 (1995).
Wenke, K. et al. Simvastatin reduces graft vessel disease and mortality after heart transplantation: a four-year randomized trial. Circulation 96, 1398?1402 (1997).
Buchwald, H., Campos, C. T., Boen, J. R., Nguyen, P. A. & Williams, S. E. Disease-free intervals after partial ileal bypass in patients with coronary heart disease and hypercholesterolemia: report from the Program on the Surgical Control of the Hyperlipidemias (POSCH). J. Am. Coll. Cardiol. 26, 351?357 (1995).
Schonbeck, U. & Libby, P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation 109, II18?II26 (2004).
Schwartz, G. G. et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 285, 1711?1718 (2001).
Anderson, T. J. et al. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N. Engl. J. Med. 332, 488?493 (1995).
Treasure, C. B. et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N. Engl. J. Med. 332, 481?487 (1995). Two seminal papers demonstrating the beneficial effects of lipid lowering in human endothelial function in patients with coronary artery disease.
Wilson, S. H. et al. Simvastatin preserves coronary endothelial function in hypercholesterolemia in the absence of lipid lowering. Arterioscler. Thromb. Vasc. Biol. 21, 122?128 (2001).
Bonetti, P. O. et al. Simvastatin preserves myocardial perfusion and coronary microvascular permeability in experimental hypercholesterolemia independent of lipid lowering. J. Am. Coll. Cardiol. 40, 546?554 (2002).
Williams, J. K., Sukhova, G. K., Herrington, D. M. & Libby, P. Pravastatin has cholesterol-lowering independent effects on the artery wall of atherosclerotic monkeys. J. Am. Coll. Cardiol. 31, 684?691 (1998).
Jarvisalo, M. J. et al. HMG CoA reductase inhibitors are related to improved systemic endothelial function in coronary artery disease. Atherosclerosis 147, 237?42 (1999).
Dupuis, J., Tardif, J. C., Cernacek, P. & Theroux, P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (reduction of cholesterol in ischemia and function of the endothelium) trial. Circulation 99, 3227?3233 (1999).
Landmesser, U. et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 111, 2356?2363 (2005).
Katznelson, S. et al. The effect of pravastatin on acute rejection after kidney transplantation-a pilot study. Transplantation 61, 1469?1474 (1996).
Holdaas, H. et al. Effect of fluvastatin on acute renal allograft rejection: a randomized multicenter trial. Kidney Int. 60, 1990?1997 (2001).
Kwak, B., Mulhaupt, F., Myit, S. & Mach, F. Statins as a newly recognized type of immunomodulator. Nature Med. 6, 1399?1402 (2000).
Ridker, P. M., Wilson, P. W. & Grundy, S. M. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation 109, 2818?2825 (2004).
Ridker, P. M., Rifai, N., Pfeffer, M. A., Sacks, F. & Braunwald, E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 100, 230?235 (1999). This study provided the first evidence that statin therapy lowered levels of the inflammatory biomarker CRP.
Ridker, P. M. et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N. Engl. J. Med. 344, 1959?1965 (2001). Clinical demonstration that healthy patients with low cholesterol but elevated levels of CRP are likely to benefit from statin therapy.
Ridker, P. M., Rifai, N. & Lowenthal, S. P. Rapid reduction in C-reactive protein with cerivastatin among 785 patients with primary hypercholesterolemia. Circulation 103, 1191?1193 (2001).
Albert, M. A., Danielson, E., Rifai, N. & Ridker, P. M. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 286, 64?70 (2001).
Jialal, I. & Devaraj, S. Antioxidants and atherosclerosis: don't throw out the baby with the bath water. Circulation 107, 926?928 (2003).
Ballantyne, C. M. et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 107, 2409?2415 (2003).
Ridker, P. M. et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation 98, 839?844 (1998). The initial description that clinical event reduction with statin therapy is linked to CRP levels.
Walter, D. H. et al. Preprocedural C-reactive protein levels and cardiovascular events after coronary stent implantation. J. Am. Coll. Cardiol. 37, 839?846 (2001).
Walter, D. H. et al. Statin therapy, inflammation and recurrent coronary events in patients following coronary stent implantation. J. Am. Coll. Cardiol. 38, 2006?2012 (2001).
Ridker, P. M. et al. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 352, 20?28 (2005). Recent data in high-risk patients demonstrating that the clinical benefit of statin therapy is linked as much to reduction in CRP as to reduction in LDL cholesterol.
Nissen, S. E. et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 352, 29?38 (2005). Recent data in stable coronary patients indicating that atherothrombotic disease regression is associated with CRP reduction.
Arnaud, C. et al. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler. Thromb. Vasc. Biol. 25, 1231?1216 (2005).
Gimbrone, M. A., Jr. Vascular endothelium, hemodynamic forces, and atherogenesis. [comment]. Am. J. Pathol. 155, 1?5 (1999).
Ignarro, L. J., Buga, G. M., Wood, K. S., Byrns, R. E. & Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl Acad. Sci. USA 84, 9265?9269 (1987).
Garg, U. C. & Hassid, A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J. Clin. Invest 83, 1774?1777 (1989).
Gauthier, T. W., Scalia, R., Murohara, T., Guo, J. P. & Lefer, A. M. Nitric oxide protects against leukocyte-endothelium interactions in the early stages of hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 15, 1652?1659 (1995).
Radomski, M. W., Rees, D. D., Dutra, A. & Moncada, S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br. J. Pharmacol. 107, 745?749 (1992).
Radomski, M. W., Palmer, R. M. & Moncada, S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 2, 1057?1058 (1987).
Matsushita, K. et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 115, 139?150 (2003).
Morrell, C. N. et al. Regulation of platelet granule exocytosis by S-nitrosylation. Proc. Natl Acad. Sci. USA 102, 3782?3787 (2005).
Yamakuchi, M. et al. HMG-CoA reductase inhibitors inhibit endothelial exocytosis and decrease myocardial infarct size. Circ. Res. 96, 1185?1192 (2005).
Tamai, O. et al. Single LDL apheresis improves endothelium-dependent vasodilatation in hypercholesterolemic humans. Circulation 95, 76?82 (1997).
O'Driscoll, G., Green, D. & Taylor, R. R. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation 95, 1126?1131 (1997).
Aikawa, M. et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation 103, 276?283 (2001).
Crisby, M. et al. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 103, 926?933 (2001).
Fukumoto, Y. et al. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of watanabe heritable hyperlipidemic rabbits. Circulation 103, 993?999 (2001).
Esmon, C. T. Inflammation and thrombosis. J. Thromb. Haemost. 1, 1343?1348 (2003).
Shi, J. et al. Statins increase thrombomodulin expression and function in human endothelial cells by a nitric oxide-dependent mechanism and counteract tumor necrosis factor α-induced thrombomodulin downregulation. Blood Coagul. Fibrinolysis 14, 575?585 (2003).
Morikawa, S. et al. The effect of statins on mRNA levels of genes related to inflammation, coagulation, and vascular constriction in HUVEC. Human umbilical vein endothelial cells. J. Atheroscler. Thromb. 9, 178?183 (2002).
Morikawa, S. et al. Global analysis of RNA expression profile in human vascular cells treated with statins. J. Atheroscler. Thromb. 11, 62?72 (2004).
Bustos, C. et al. HMG-CoA reductase inhibition by atorvastatin reduces neointimal inflammation in a rabbit model of atherosclerosis. J. Am. Coll. Cardiol. 32, 2057?2064 (1998).
Romano, M. et al. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab. Invest. 80, 1095?1100 (2000).
Diomede, L. et al. In vivo anti-inflammatory effect of statins is mediated by nonsterol mevalonate products. Arterioscler. Thromb. Vasc. Biol. 21, 1327?1332 (2001).
Ito, T., Ikeda, U., Yamamoto, K. & Shimada, K. Regulation of interleukin-8 expression by HMG-CoA reductase inhibitors in human vascular smooth muscle cells. Atherosclerosis 165, 51?55 (2002).
Simon, D. I. et al. Decreased neointimal formation in Mac-1(−/−) mice reveals a role for inflammation in vascular repair after angioplasty. J. Clin. Invest. 105, 293?300 (2000).
Weber, C., Erl, W., Weber, K. S. & Weber, P. C. HMG-CoA reductase inhibitors decrease CD11b expression and CD11b-dependent adhesion of monocytes to endothelium and reduce increased adhesiveness of monocytes isolated from patients with hypercholesterolemia. J. Am. Coll. Cardiol. 30, 1212?1217 (1997).
Weitz-Schmidt, G. et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nature Med. 7, 687?692 (2001).
Chen, Z. et al. Simvastatin reduces neointimal thickening in low-density lipoprotein receptor-deficient mice after experimental angioplasty without changing plasma lipids. Circulation 106, 20?23 (2002).
Ehrenstein, M. R., Jury, E. C. & Mauri, C. Statins for atherosclerosis--as good as it gets? N. Engl. J. Med. 352, 73?75 (2005).
Laufs, U., Marra, D., Node, K. & Liao, J. K. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1). J. Biol. Chem. 274, 21926?2131 (1999).
Yang, Z. et al. HMG-CoA reductase inhibition improves endothelial cell function and inhibits smooth muscle cell proliferation in human saphenous veins. J. Am. Coll. Cardiol. 36, 1691?1697 (2000).
Guijarro, C. et al. 3-Hydroxy-3-methylglutaryl coenzyme a reductase and isoprenylation inhibitors induce apoptosis of vascular smooth muscle cells in culture. Circ. Res. 83, 490?500 (1998).
Fitzgerald, D. J., Roy, L., Catella, F. & FitzGerald, G. A. Platelet activation in unstable coronary disease. N. Engl. J. Med. 315, 983?989 (1986).
Vaughan, C. J., Gotto, A. M., Jr. & Basson, C. T. The evolving role of statins in the management of atherosclerosis. J. Am. Coll. Cardiol. 35, 1?10 (2000).
Lijnen, P., Echevaria-Vazquez, D. & Petrov, V. Influence of cholesterol-lowering on plasma membrane lipids and function. Methods Find. Exp. Clin. Pharmacol. 18, 123?136 (1996).
Alfon, J., Pueyo Palazon, C., Royo, T. & Badimon, L. Effects of statins in thrombosis and aortic lesion development in a dyslipemic rabbit model. Thromb Haemost 81, 822?827 (1999).
Van Aelst, L. & D'Souza-Schorey, C. Rho GTPases and signaling networks. Genes Dev. 11, 2295?322 (1997).
Liao, J. K. & Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 45, 89?118 (2005).
Liu, L. et al. Integrin-dependent leukocyte adhesion involves geranylgeranylated protein(s). J. Biol. Chem. 274, 33334?33340 (1999).
Rasmussen, L. M. et al. Diverse effects of inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase on the expression of VCAM-1 and E-selectin in endothelial cells. Biochem. J. 360, 363?370 (2001).
Yoshida, M. et al. Hmg-CoA reductase inhibitor modulates monocyte-endothelial cell interaction under physiological flow conditions in vitro: involvement of Rho GTPase-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 21, 1165?1171 (2001).
Laufs, U., La Fata, V., Plutzky, J. & Liao, J. K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 97, 1129?1135 (1998).
Pagano, P. J. et al. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc. Natl Acad. Sci. USA 94, 14483?14488 (1997).
Gorlach, A. et al. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ. Res. 87, 26?32 (2000).
Wassmann, S. et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 22, 300?305 (2002).
Marchesi, S. et al. Short-term atorvastatin treatment improves endothelial function in hypercholesterolemic women. J. Cardiovasc. Pharmacol. 36, 617?621 (2000).
John, S. et al. Rapid improvement of nitric oxide bioavailability after lipid-lowering therapy with cerivastatin within two weeks. J. Am. Coll. Cardiol. 37, 1351?1358 (2001).
Tsunekawa, T. et al. Cerivastatin, a hydroxymethylglutaryl coenzyme a reductase inhibitor, improves endothelial function in elderly diabetic patients within 3 days. Circulation 104, 376?379 (2001).
Thakur, N. K. et al. HMG-CoA reductase inhibitor stabilizes rabbit atheroma by increasing basal NO and decreasing superoxide. Am. J. Physiol. Heart Circ. Physiol. 281, H75?H83 (2001).
Rikitake, Y. et al. Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 154, 87?96 (2001).
Collins, T. & Cybulsky, M. I. NF-kB: pivotal mediator or innocent bystander in atherogenesis? J. Clin. Invest. 107, 255?264 (2001).
Hayden, M. S. & Ghosh, S. Signaling to NF-κB. Genes Dev 18, 2195?2224 (2004).
Shaulian, E. & Karin, M. AP-1 in cell proliferation and survival. Oncogene 20, 2390?2400 (2001).
Montaner, S., Perona, R., Saniger, L. & Lacal, J. C. Activation of serum response factor by RhoA is mediated by the nuclear factor-κB and C/EBP transcription factors. J. Biol. Chem. 274, 8506?8515 (1999).
Perona, R. et al. Activation of the nuclear factor-κB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 11, 463?475 (1997).
Chang, J. H., Pratt, J. C., Sawasdikosol, S., Kapeller, R. & Burakoff, S. J. The small GTP-binding protein Rho potentiates AP-1 transcription in T cells. Mol. Cell. Biol. 18, 4986?4993 (1998).
Colli, S. et al. Vastatins inhibit tissue factor in cultured human macrophages. A novel mechanism of protection against atherothrombosis. Arterioscler. Thromb. Vasc. Biol. 17, 265?272 (1997).
Meroni, P. L. et al. Statins prevent endothelial cell activation induced by antiphospholipid (anti-β2-glycoprotein I) antibodies: effect on the proadhesive and proinflammatory phenotype. Arthritis Rheum. 44, 2870?2878 (2001).
Hernandez-Presa, M. A. et al. Simvastatin reduces NF-κB activity in peripheral mononuclear and in plaque cells of rabbit atheroma more markedly than lipid lowering diet. Cardiovasc. Res. 57, 168?177 (2003).
Dichtl, W. et al. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 23, 58?63 (2003).
Wagner, A. H., Gebauer, M., Guldenzoph, B. & Hecker, M. 3-hydroxy-3-methylglutaryl coenzyme A reductase-independent inhibition of CD40 expression by atorvastatin in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 22, 1784?1789 (2002).
Feinberg, M. W., Lin, Z., Fisch, S. & Jain, M. K. An emerging role for kruppel-like factors in vascular biology. Trends Cardiovasc. Med. 14, 241?246 (2004).
Plutzky, J. Peroxisome proliferator-activated receptors as therapeutic targets in inflammation. J. Am. Coll. Cardiol. 42, 1764?1746 (2003).
Anderson, K. P., Kern, C. B., Crable, S. C. & Lingrel, J. B. Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Kruppel-like factor: identification of a new multigene family. Mol. Cell. Biol. 15, 5957?5965 (1995).
Wani, M. A., Wert, S. E. & Lingrel, J. B. Lung Kruppel-like factor, a zinc finger transcription factor, is essential for normal lung development. J. Biol. Chem. 274, 21180?21185 (1999).
Kuo, C. T., Veselits, M. L. & Leiden, J. M. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science 277, 1986?1990 (1997).
Kuo, C. T. et al. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 11, 2996?3006 (1997). This paper identified LKLF/KLF2 as an essential regulator of vessel development.
Dekker, R. J. et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100, 1689?1698 (2002). Identification of KLF2 as a shear stress response gene.
SenBanerjee, S. et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J. Exp. Med. 199, 1305?1315 (2004). The first paper to identify KLF2 as an inducer of eNOS and inhibitor of cytokine activation of the endothelium.
Lin, Z. et al. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ. Res. 96, e48?e57 (2005).
Kumar, A., Lin, Z., Senbanerjee, S. & Jain, M. K. Tumor necrosis factor α-mediated reduction of KLF2 is due to inhibition of MEF2 by NF-κB and histone deacetylases. Mol. Cell. Biol. 25, 5893?5903 (2005).
Buckley, A. F., Kuo, C. T. & Leiden, J. M. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc--dependent pathway. Nature Immunol. 2, 698?704 (2001).
Parmar, K. M. et al. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J. Biol. Chem. 280, 26714?26719 (2005).
Sen-Banerjee, S. et al. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 112, 720?726 (2005). This paper identifies KLF2 as essential for statin mediated effects in endothelial cells and links KLF2 to the Rho pathway.
Marx, N., Libby, P. & Plutzky, J. Peroxisome proliferator-activated receptors (PPARs) and their role in the vessel wall: possible mediators of cardiovascular risk? J. Cardiovasc. Risk 8, 203?210 (2001).
Ricote, M., Li, A. C., Willson, T. M., Kelly, C. J. & Glass, C. K. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391, 79?82 (1998).
Li, A. C. et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ. J. Clin. Invest. 114, 1564?1576 (2004).
Zelvyte, I., Dominaitiene, R., Crisby, M. & Janciauskiene, S. Modulation of inflammatory mediators and PPARγ and NFκB expression by pravastatin in response to lipoproteins in human monocytes in vitro. Pharmacol. Res. 45, 147?154 (2002).
Landrier, J. F. et al. Statin induction of liver fatty acid-binding protein (L-FABP) gene expression is peroxisome proliferator-activated receptor-α-dependent. J. Biol. Chem. 279, 45512?45518 (2004).
Inoue, I. et al. Fibrate and statin synergistically increase the transcriptional activities of PPARα /RXRα and decrease the transactivation of NFκB. Biochem. Biophys. Res. Commun. 290, 131?139 (2002).
Martin, G. et al. Statin-induced inhibition of the Rho-signaling pathway activates PPARα and induces HDL apoA-I. J. Clin. Invest. 107, 1423?1432 (2001). An important paper linking statins to the PPAR pathway.
Ridker, P. M. et al. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: an analysis of the PROVE-IT TIMI-22 trial. J. Am. Coll. Cardiol. 45, 1644?1648 (2005).
Ridker, P. M. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation 108, 2292?2297 (2003).
Chasman, D. I. et al. Pharmacogenetic study of statin therapy and cholesterol reduction. JAMA 291, 2821?2827 (2004).
Poynter, J. N. et al. Statins and the risk of colorectal cancer. N. Engl. J. Med. 352, 2184?92 (2005).
Mueller, B. K., Mack, H. & Teusch, N. Rho kinase, a promising drug target for neurological disorders. Nature Rev. Drug Discov. 4, 387?398 (2005).
Shimokawa, H. & Takeshita, A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler. Thromb. Vasc. Biol. 25, 1767?1775 (2005).
Shimokawa, H. et al. Anti-anginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: a multicenter study. J. Cardiovasc. Pharmacol. 40, 751?761 (2002).
Acknowledgements
P.M.R. receives additional support from a National Institute of Health Pharmacogenetics and Risk of Cardiovascular Disease (PARC) grant. M.K.J. is supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
P.M.R. is listed as a co-inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease, and has also received investigator-initiated research grant support from manufacturers of statin agents.
Glossary
- BALLOON ANGIOPLASTY
-
A medical procedure in which a balloon-tip catheter is inflated inside an artery to open narrowed or blocked blood vessels of the heart (coronary arteries), stretching the intima and leaving a ragged interior surface after deflation, which triggers a healing response and breaking up of plaque.
- STENTING
-
Usually follows angioplasty; a wire mesh tube is placed in a damaged artery to support the arterial walls and keep them open.
- AUTOCRINE
-
Describing an agent secreted from a cell that acts on the cell in which it is produced.
- PARACRINE
-
Describing an agent secreted from a cell that acts on other cells in the local environment.
- ISCHAEMIA
-
A decrease in the blood supply to a bodily organ or tissue caused by constriction or obstruction of the blood.
- HYPERLIPIDAEMIA
-
An excess of lipids, either triglycerides or cholesterol, in the blood. Lipids circulate as free or esterified entities in lipoprotein particles.
- HAEMOSTATIC BALANCE
-
The balance between coagulation and fibrinolysis, maintained by a dynamic process involving fibrinolytic activators, inhibitors and cellular elements such as platelet cytoskeleton, platelet cytoplasmic granules, and platelet cell surfaces.
- CAROTID ENDARTERECTOMY
-
Procedure in which an artery is opened and a portion of atherosclerotic disease (plaque) is removed.
- LIPID RAFT
-
A cholesterol-rich micro-domain within a cell membrane.
- PLATELET HYPER-REACTIVITY
-
Enhanced platelet aggregation and adhesion activity that can be observed in conditions such as inflammation.
- ISOPRENOIDS
-
A large and diverse class of naturally occurring lipids derived from five-carbon isoprene units formed during cholesterol biosynthesis.
Rights and permissions
About this article
Cite this article
Jain, M., Ridker, P. Anti-Inflammatory Effects of Statins: Clinical Evidence and Basic Mechanisms. Nat Rev Drug Discov 4, 977–987 (2005). https://doi.org/10.1038/nrd1901
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd1901
This article is cited by
-
Association between statin use and the risk for idiopathic pulmonary fibrosis and its prognosis: a nationwide, population-based study
Scientific Reports (2024)
-
The role of systemic statins in the inception and healing of apical periodontitis: a systematic review
BMC Oral Health (2023)
-
Phenotypic screening platform identifies statins as enhancers of immune cell-induced cancer cell death
BMC Cancer (2023)
-
Sex-specific risk factors associated with graves’ orbitopathy in Korean patients with newly diagnosed graves’ disease
Eye (2023)
-
Bile acid binds heart failure and inflammation
Nature Metabolism (2023)