Key Points
-
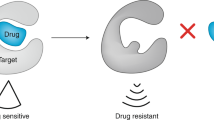
The success of kinase inhibitors, such as imatinib and gefitinib, has shown that the development of specific, targeted therapies for cancer is possible.
-
However, there have been many cases of drug resistance to imatinib observed in the clinic and this has consequences for the development of second-generation kinase inhibitors.
-
Current efforts are focused on characterizing the structural determinants of imatinib resistance observed in the clinic. These studies illustrate the importance of features such as the gatekeeper residue, the p-loop and the activation loop of protein kinases.
-
The design of more effective inhibitors based on this structural knowledge, combined with the development of multi-targeted kinase inhibitors that show improved efficacy, hold great promise for cancer therapy.
Abstract
Selective inhibition of protein tyrosine kinases is gaining importance as an effective therapeutic approach for the treatment of a wide range of human cancers. However, as extensively documented for the BCR–ABL oncogene in imatinib-treated leukaemia patients, clinical resistance caused by mutations in the targeted oncogene has been observed. Here, we look at how structural and mechanistic insights from imatinib-insensitive Bcr–Abl have been exploited to identify second-generation drugs that override acquired target resistance. These insights have created a rationale for the development of either multi-targeted protein kinase inhibitors or cocktails of selective antagonists as antitumour drugs that combine increased therapeutic potency with a reduced risk of the emergence of molecular resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Blume-Jensen, P. & Hunter, T. Oncogenic kinase signalling. Nature 411, 355–365 (2001).
Kaelin, W. G. Jr. Gleevec: prototype or outlier? Sci. STKE 2004, PE12 (2004).
Cohen, P. Protein kinases — the major drug targets of the twenty-first century? Nature Rev. Drug Discov. 1, 309–315 (2002).
Dancey, J. & Sausville, E. A. Issues and progress with protein kinase inhibitors for cancer treatment. Nature Rev. Drug Discov. 2, 296–313 (2003).
Gschwind, A., Fischer, O. M. & Ullrich, A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nature Rev. Cancer 4, 361–370 (2004).
Coates, A., Hu, Y., Bax, R. & Page C. The future challenges facing the development of new antimicrobial drugs. Nature Rev. Drug Discov. 1, 895–910 (2002).
Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 406, 775–781 (2000).
von Bubnoff, N., Peschel, C. & Duyster, J. Resistance of Philadelphia-chromosome positive leukemia towards the kinase inhibitor imatinib (STI571, Glivec): a targeted oncoprotein strikes back. Leukemia 17, 829–838 (2003).
Cowan-Jacob, S. W. et al. Imatinib (STI571) resistance in chronic myelogenous leukemia: molecular basis of the underlying mechanisms and potential strategies for treatment. Mini Rev. Med. Chem. 4, 285–299 (2004).
Hochhaus, A. & La Rosee, P. Imatinib therapy in chronic myelogenous leukemia: strategies to avoid and overcome resistance. Leukemia 18, 1321–1331 (2004).
Nardi, V., Azam, M. & Daley, G. Q. Mechanisms and implications of imatinib resistance mutations in BCR–ABL. Curr. Opin. Hematol. 11, 35–43 (2004).
Ross, D. M. & Hughes, T. P. Cancer treatment with kinase inhibitors: what have we learnt from imatinib? Br. J. Cancer 90, 12–19 (2004).
Capdeville, R., Buchdunger, E., Zimmermann, J. & Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nature Rev. Drug Discov. 1, 493–502 (2002).
Faderl, S. et al. The biology of chronic myeloid leukemia. N. Engl. J. Med. 341, 164–172 (1999).
Sawyers, C. L. Chronic myeloid leukaemia. N. Engl. J. Med. 340, 1330–1340 (1999).
Daley, G. Q., van Etten, R. A. & Baltimore, D. Induction of chronic myelogenous leukemia in mice by the p210Bcr/Abl gene of the Philadelphia chromosome. Science 247, 824–830 (1990).
Lugo, T. G. et al. Tyrosine kinase activity and transformation potency of Bcr–Abl oncogene products. Science 247, 1079–1082 (1990).
Druker, B. G. et al. Activity of a specific inhibitor of the BCR–ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344, 1038–1042 (2001).
Druker, B. J. et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr–Abl positive cells. Nature Med. 2, 561–566 (1996).
Ottmann, O. G. et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood 100, 1965–1971 (2002).
Druker, B. J. Imatinib as a paradigm of targeted therapies. Adv. Cancer Res. 91, 1–30 (2004).
Hingorani, S. R. & Tuveson, D. A. Targeting oncogene dependence and resistance. Cancer Cell 3, 414–417 (2003).
Gambacorti-Passerini, C. et al. α1 acid glycoprotein binds to imatinib (STI571) and substantially alters its pharmacokinetics in chronic myeloid leukemia patients. Clin. Cancer Res. 9, 625–632 (2003).
Mahon, F. X. et al. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood 101, 2368–2373 (2003).
Thomas, J., Wang, L., Clark, R. E. & Pirmohamed, M. Active transport of imatinib into and out of cells: Implications for drug resistance. Blood 17 Aug 2004 (doi:10.1182/blood-2003-12-4276).
Donato, N. J. et al. BCR–ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood 101, 690–698 (2003).
Dai, Y., Rahmani, M., Corey, S. J., Dent, P. & Grant, S. A Bcr/Abl-independent, Lyn-dependent form of imatinib mesylate (STI-571) resistance is associated with altered expression of Bcl-2. J. Biol. Chem. 279, 34227–34239 (2004).
Gorre, M. E. et al. Clinical resistance to STI-571 cancer therapy caused by BCR–ABL gene mutation or amplification. Science 293, 876–880 (2001).
Graham, S. M. et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 99, 319–325 (2002).
Branford, S. et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood 99, 3472–3475 (2002).
Hofmann, W. K. et al. Ph(+) acute lymphoblastic leukemia resistant to the tyrosine kinase inhibitor STI571 has a unique BCR–ABL gene mutation. Blood 99, 1860–1862 (2002).
Roumiantsev, S. et al. Clinical resistance to the kinase inhibitor STI-571 in chronic myeloid leukemia by mutation of Tyr-253 in the Abl kinase domain P-loop. Proc. Natl Acad. Sci. USA 99, 10700–10705 (2002).
Shah, N. P. et al. Multiple BCR–ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2, 117–125 (2002). An excellent paper about the amino-acid substitutions in Bcr–Abl from relapsed CML patients and the potential mechanisms how these mutations confer imatinib resistance.
von Bubnoff, N., Schneller, F., Peschel, C. & Duyster, J. BCR–ABL gene mutations in relation to clinical resistance of Philadelphia-chromosome-positive leukaemia to STI571: a prospective study. Lancet 359, 487–491 (2002).
Corbin, A. S., La Rosee, P., Stoffregen, E. P., Druker, B. J. & Deininger, M. W. Several Bcr–Abl kinase domain mutants associated with imatinib mesylate resistance remain sensitive to imatinib. Blood 101, 4611–4614 (2003).
Schindler, T. et al. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 289, 1938–1942 (2000). First report of the co-crystal structure of an imatinib analogue in complex with the tyrosine kinase Abl.
Blencke, S. et al. Characterization of a conserved structural determinant controlling protein kinase sensitivity to selective inhibitors. Chem. Biol. 11, 691–791 (2004). This study provides an analysis of the 'gatekeeper' residue in several tyrosine kinases and shows the general relevance of this site for resistance formation against small-molecule inhibitors.
Nagar, B. et al. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571). Cancer Res. 62, 4236–4243 (2002).
Branford, S. et al. Detection of BCR–ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood 102, 276–832 (2003). This study shows that the type of the imatinib resistance-inducing mutation in BCR–ABL predicts the clinical prognosis for relapsed CML patients.
Azam, M., Latek, R. R. & Daley, G. Q. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR–ABL. Cell 112, 831–843 (2003). This paper describes an interesting screening technique to identify potential mechanisms of resistance to targeted kinase inhibitors.
Apperley, J. F. et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N. Engl. J. Med. 347, 481–487 (2002).
Cools, J. et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N. Engl. J. Med. 348, 1201–1214 (2003). This study identifies a constitutively active PDGFRα variant as imatinib target in a haematologic disorder and further reports the emergence of imatinib resistance as a consequence of a mutation affecting the PDGFRα residue homologous to Thr315 in Abl.
Demetri, G. D. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480 (2002).
Tamborini, E. et al. A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology 127, 294–299 (2004).
Wakai, T. et al. Late resistance to imatinib therapy in a metastatic gastrointestinal stromal tumour is associated with a second KIT mutation. Br. J. Cancer 90, 2059–2061 (2004).
Ma, Y. et al. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood 99, 1741–1744 (2002).
Wisniewski, D. et al. Characterization of potent inhibitors of the Bcr–Abl and the c-kit receptor tyrosine kinases. Cancer Res. 62, 4244–4255 (2002).
La Rosée, P., Corbin, A. S., Stoffregen, E. P., Deininger, M. W. & Druker, B. J. Activity of the Bcr–Abl kinase inhibitor PD180970 against clinically relevant Bcr–Abl isoforms that cause resistance to imatinib mesylate (Gleevec, STI571). Cancer Res. 62, 7149–7153 (2002). This is the first report demonstrating that many imatinib-restistant Bcr–Abl variants retain sensitivity to a structurally distinct kinase inhibitor.
Huron, D. R. et al. A novel pyridopyrimidine inhibitor of Abl kinase is a picomolar inhibitor of Bcr–Abl-driven K562 cells and is effective against STI571-resistant Bcr–Abl mutants. Clin. Cancer Res. 9, 1267–1273 (2003).
von Bubnoff, N. et al. Inhibition of wild-type and mutant Bcr–Abl by pyrido-pyrimidine-type small molecule kinase inhibitors. Cancer Res. 63, 6395–6404 (2003).
Kantarjian, H. M. et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood 101, 473–475 (2003).
Shah, N. P. et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 305, 399–401 (2004).
O'Hare, T. et al. Inhibition of wild-type and mutant Bcr–Abl by AP23464, a potent ATP-based oncogenic protein kinase inhibitor: Implications for CML. Blood 104, 2532–2539 (2004).
Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002).
Eyers, P. A., Craxton, M., Morrice, N., Cohen, P. & Goedert, M. Conversion of SB 203580-insensitive MAP kinase family members to drug-sensitive forms by a single amino-acid substitution. Chem. Biol. 5, 321–328 (1998).
Liu, Y. et al. Structural basis for selective inhibition of Src family kinases by PP1. Chem. Biol. 8, 257–266 (1999).
Blencke, S., Ullrich, A. & Daub, H. Mutation of threonine 766 in the epidermal growth factor receptor reveals a hotspot for resistance formation against selective tyrosine kinase inhibitors. J. Biol. Chem. 278, 15435–15440 (2003).
Stamos, J., Sliwkowski, M. X. & Eigenbrot, C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem. 277, 46265–46272 (2002).
Muhsin, M., Graham, J. & Kirkpatrick, P. Gefitinib. Nature Rev. Drug Discov. 2, 515–516 (2003).
Cohen, M. H. et al. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin. Cancer Res. 10, 1212–1218 (2004).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004). This paper and reference 62 were the first to describe a correlation of drug-sensitizing, activating mutations in the EGFR gene with clinical responses to gefitinib in lung cancer patients.
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Pao, W. et al. EGF receptor gene mutations are common in lung cancers from 'never smokers' and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl Acad. Sci. USA 101, 13306–13311 (2004).
Sordella, R., Bell, D. W., Haber, D. A. & Settleman, J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 305, 1163–1167 (2004).
Cools, J. et al. PKC412 overcomes resistance to imatinib in a murine model of FIP1L1-PDGFRα-induced myeloproliferative disease. Cancer Cell 3, 459–469 (2003).
Mohammadi, M. et al. Structure of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276, 955–960 (1997).
Roche-Lestienne, C. et al. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood 100, 1014–1018 (2002).
Roche-Lestienne, C., Lai, J. L., Darre, S., Facon T. & Preudhomme, C. A mutation conferring resistance to imatinib at the time of diagnosis of chronic myelogenous leukemia. N. Engl. J. Med. 348, 2265–2266 (2003).
Hofmann, W. K. et al. Presence of the BCR–ABL mutation Glu255Lys prior to STI571 (imatinib) treatment in patients with Ph+ acute lymphoblastic leukemia. Blood 102, 659–661 (2003).
Druker, B. J. Overcoming resistance to imatinib by combining targeted agents. Mol. Cancer Ther. 2, 225–226 (2003).
Hampton, T. 'Promiscuous' anticancer drugs that hit multiple targets may thwart resistance. JAMA 292, 419–22 (2004).
Morphy, R., Kay, C. & Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today 9, 641–651 (2004).
Fong, T. A. et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 59, 99–106 (1999).
Laird, A. D. et al. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res. 60, 4152–4162 (2000).
Bergers, G., Song, S., Meyer-Morse, N., Bergsland, E. & Hanahan, D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 111, 1287–1295 (2003).
Eskens, F. A. Angiogenesis inhibitors in clinical development; where are we now and where are we going? Br. J. Cancer 90, 1–7 (2004).
Abrams, T. J., Lee, L. B., Murray, L. J., Pryer, N. K. & Cherrington, J. M. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol. Cancer Ther. 2, 471–478 (2003).
Mendel, D. B. et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 9, 327–337 (2003).
O'Farrell, A. M. et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 101, 3597–3605 (2003).
Schueneman, A. J. et al. SU11248 maintenance therapy prevents tumor regrowth after fractionated irradiation of murine tumor models. Cancer Res. 63, 4009–4016 (2003).
Davies, S. P., Reddy, H., Caivano, M. & Cohen, P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 (2000).
Bain, J., McLauchlan, H., Elliott, M. & Cohen, P. The specificities of protein kinase inhibitors: an update. Biochem. J. 371, 199–204 (2003).
Godl, K. et al. An efficient proteomics method to identify the cellular targets of protein kinase inhibitors. Proc. Natl Acad. Sci. USA 100, 15434–15439 (2003).
Brehmer, D., Godl, K., Zech, B., Wissing, J. & Daub, H. Proteome-wide identification of cellular targets affected by bisindolylmaleimide-type protein kinase C inhibitors. Mol. Cell. Proteomics 3, 490–500 (2004).
Daub, H., Godl, K., Brehmer, D., Klebl, B. & Müller, G. Evaluation of kinase inhibitor selectivity by chemical proteomics. Assay Drug. Dev. Technol. 2, 215–224 (2004).
Cheok, M. H. et al. Treatment-specific changes in gene expression discriminate in vivo drug response in human leukemia cells. Nature Genet. 34, 85–90 (2003).
Pack, S. D. et al. Simultaneous suppression of epidermal growth factor receptor and c-erbB-2 reverses aneuploidy and malignant phenotype of a human ovarian carcinoma cell line. Cancer Res. 64, 789–794 (2004). This interesting paper reports the reversal of aneuploidy after co-targeting of the EGFR and the closely related HER2 tyrosine kinase.
Baselga, J. & Hammond, L. A. HER-targeted tyrosine-kinase inhibitors. Oncology 63 (Suppl. 1), 6–16 (2002).
Towatari, M. et al. Combination of intensive chemotherapy and imatinib can rapidly induce high-quality complete remission for a majority of patients with newly diagnosed BCR–ABL positive acute lymphoblastic leukemia. Blood 17 Aug 2004 (doi:182/blood-2004-04–1389)
Hoover, R. R., Mahon, F. X., Melo, J. V. & Daley, G. Q. Overcoming STI571 resistance with the farnesyl transferase inhibitor SCH66336. Blood 100, 1068–1071 (2002).
Gorre, M. E., Ellwood-Yen, K., Chiosis, G., Rosen, N. & Sawyers, C. L. BCR–ABL point mutants isolated from patients with imatinib mesylate-resistant chronic myeloid leukemia remain sensitive to inhibitors of the BCR–ABL chaperone heat shock protein 90. Blood 100, 3041–3044 (2002).
Richman, D. D. HIV chemotherapy. Nature 410, 995–1001 (2001).
Acknowledgements
The authors wish to thank D. Brehmer for stimulating discussions and his contributions to the illustrations in figure 2 and figure 5. The work carried out in the laboratory of H.D. is supported by a grant from the German Ministry for Education and Research (Bundesministerium für Bildung und Forschung, BMBF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
H.D. is an employee of Axxima Pharmaceuticals AG.A.U. and H.D. are shareholders in Axxima Pharmaceuticals AG.
Glossary
- CHRONIC MYELOID LEUKAEMIA
-
(CML). A myeloproliferative disorder that is characterized by a distinctive cytogenetic abnormality, the Philadelphia (Ph) chromosome.
- BCR–ABL
-
The fusion gene that results from the chromosomal translocation that causes the Abelson protein tyrosine kinase gene to fuse with the BCR gene on the so-called Philadelphia (Ph) chromosome.
- BLAST CRISIS
-
The aggressive phase of chronic myelogenous leukaemia evidenced by an increased number of immature white blood cells in the circulating blood.
- INDUCED-FIT MECHANISM
-
The interaction between a protein and ligand in which the binding of the ligand alters the conformation of the protein's active site to best accommodate binding of the ligand.
- IDIOPATHIC HYPER-EOSINOPHILIC SYNDROME
-
The presence of prolonged eosinophilia without an identifiable underlying cause and with evidence of end-organ dysfunction.
- INTERSTITIAL CHROMOSOMAL DELETION
-
Loss of material from within one of the chromosome arms.
- HUMAN KINOME
-
The collection of genes in the human genome encoding kinases.
- DUAL-SPECIFICITY KINASE INHIBITOR
-
An inhibitor that specifically interferes with two distinct protein kinase activities.
Rights and permissions
About this article
Cite this article
Daub, H., Specht, K. & Ullrich, A. Strategies to overcome resistance to targeted protein kinase inhibitors. Nat Rev Drug Discov 3, 1001–1010 (2004). https://doi.org/10.1038/nrd1579
Issue Date:
DOI: https://doi.org/10.1038/nrd1579
This article is cited by
-
Distinct resistance mechanisms arise to allosteric vs. ATP-competitive AKT inhibitors
Nature Communications (2022)
-
Facing small and biased data dilemma in drug discovery with enhanced federated learning approaches
Science China Life Sciences (2022)
-
Feedback activation of STAT3 limits the response to PI3K/AKT/mTOR inhibitors in PTEN-deficient cancer cells
Oncogenesis (2021)
-
Current Approaches to Philadelphia Chromosome–Positive B-Cell Lineage Acute Lymphoblastic Leukemia: Role of Tyrosine Kinase Inhibitor and Stem Cell Transplant
Current Oncology Reports (2021)
-
The therapeutic potential of Aurora kinases targeting in glioblastoma: from preclinical research to translational oncology
Journal of Molecular Medicine (2020)