Key Points
-
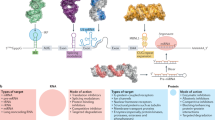
Functionalization of the human genome is expected to lead to the discovery of novel drug targets that will yield new therapeutic agents.
-
Gene-family mining and comparative genomics can help to identify potential novel targets using in silico approaches.
-
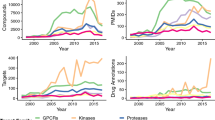
Differential RNA-expression analysis is the highest throughput method for gene functionalization and has been used in many studies to compare disease versus normal or drug-treated versus untreated cells or animals/patients. The limitation of this approach is that it is only an indirect measurement of the proteins that are the actual drug targets.
-
Proteomics is a direct analysis of the protein content, modifications and interactions. Although its throughput is not as high as that of RNA-expression analysis, it looks directly at the potential drug target and can be used to determine the target for drugs with an unknown mechanism of action.
-
Oligonucleotides are used in gene functionalization as a means of rapidly and specifically inhibiting potential targets to simulate the effects of drugs on the target. Antisense oligonucleotides, ribozymes and, most recently, RNA interference (RNAi) can all be used for such target-validation studies.
-
Genome-wide overexpression or knockdown is now possible through large collections of human cDNAs or RNAi reagents. Through automation and robotics, testing of essentially each individual gene in the genome in cell-based phenotypic or reporter assays is now possible. This will allow the building of databases of gene function to predict disease relevance and specificity as well as the potential for being an effective drug target.
-
The sequencing of a number of genomes has revealed a high degree of conservation of many genes and biological pathways across diverse species. This allows for the use of relatively simple model organisms, such as the fruitfly and zebrafish, as surrogates for humans in determining disease pathways.
Abstract
The completion of the sequencing of the human genome, and those of other organisms, is expected to lead to many potential new drug targets in various diseases, and it is predicted that novel therapeutic agents will be developed against such targets. The role of functional genomics in modern drug discovery is to prioritize these targets and to translate that knowledge into rational and reliable drug discovery. Here, we describe the field of functional genomics and review approaches that have been applied to drug discovery, including RNA profiling, proteomics, antisense and RNA interference, model organisms and high-throughput, genome-wide overexpression or knockdowns, and outline the future directions that are likely to yield new drug targets from genomics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Pearson, W. R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 183, 63–98 (1990).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Eddy, S. R. HMMER: profile hidden Markov models for protein sequence analysis [online], <http://hmmer.wustl.edu/> (2001).
Karplus, L. et al. What is the value added by human intervention in protein structure prediction? Proteins 45 (Suppl. 5) 86–91 (2001).
Smith, T. F. & Waterman, M. S. Identification of common molecular subsequences. J. Mol. Biol. 147, 195–197 (1981).
Lismaa, T. P. et al. G Protein-Coupled Receptors (Springer, New York, 1995).
Jones, C. E. et al. Expression and characterization of 5-oxo-6E, 8Z, 11Z, 14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils. Mol. Pharmacol. 63, 471–477 (2003).
Tatusov, R. L., Koonin, E. V. & Lipman, D. J. A genomic perspective on protein families. Science 278, 631–637 (1997).
Venter, J. C. et al. The sequence of the human genome. Science 291, 1304–1351 (2001).
Scheel, J. et al. Yellow pages to the transcriptome. Pharmacogenomics 3, 791–807 (2002).
Perou, C. M. et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc. Natl Acad. Sci. USA 96, 9212–9217 (1999).
Golub, T. R. et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 286, 531–537 (1999).
Ash, A. et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403, 503–511 (2000). References 12–14 were among the earliest works to look at gene-expression patterns as a way to classify cancers and to distinguish among phenotypically similar cancers. Such classification is often crucial in determining the outlook and most effective treatment for the cancer.
Scott, L. et al. Prediction of central nervous system embryonal tumor outcome based on gene expression. Nature 415, 436–442 (2002).
Yeoh, E. J. et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic lukemia by gene expression profiling. Cancer Cell 1, 133–143 (2002).
Singh, D. et al. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell 1, 203–209 (2002).
Ross, D. T. et al. Systematic variation in gene expression in patterns in human cancer cell lines. Nature Genet. 24, 227–235 (2000).
Scherf, U. et al. A gene expression database for the molecular pharmacology of cancer. Nature Genet. 24, 236–244 (2000).
Mirnics, K., Middleton, F. A., Marquez, A., Lewis, D. A. & Levitt, P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron 28, 53–67 (2000).
Hakak, Y. et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl Acad. Sci. USA 98, 4746–4751 (2001).
Middleton, F. A., Mirnics, K., Pierri, J. N., Lewis, D. A. & Levitt, P. Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J. Neurosci. 22, 2718–2729 (2002).
Vawter, P. M. et al. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophr. Res. 58, 11–20 (2002).
Hemby, S. E. et al. Gene expression profile for schizophrenia: discrete neuron transcription patterns in the entorhinal cortex. Arch. Gen. Psychiatry 59, 631–640 (2002).
Buxton, F. et al. RNA profiling in the anterior cingulated in schizophrenia. (Abstracts of the 9th International Congress on Schizophrenia Research). Schizophr. Res. 60, 95 (2003).
Saghatelian, A., Jessani, M., Joseph, A., Humphrey, M. & Cravatt, B. F. Activity-based probes for the proteomic profiling of metalloproteases. Proc. Natl Acad. Sci. USA 101, 10000–10005 (2004).
Unlu, M., Morgan, M. E. & Minden, J. S. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis 18, 2071–2077 (1997).
Gygi, S. P. et al. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nature Biotechnol. 17, 994–999 (1999).
Merchant, M. & Weinberger, S. R. Recent advancements in surface-enhanced laser desorption/ionization-time of flight-mass spectrometry. Electrophoresis 6, 1164–1177 (2000).
Taunton, J., Hassig, C. A. & Schreiber, S. L. A mammalian histone deacetylase related to the yeast transcripitonal regulator Rpd3p. Science 272, 408–411 (1996).
Petricoin, E. F. et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet 359, 572–577 (2002).
Ramachandran, N. et al. Self-assembling protein microarrays. Science 305, 86–90 (2004).
Towbin, H. et al. Proteomics-based target identification: bengamides as a new class of methionine aminopeptidase inhibitors. J. Biol. Chem. 278, 52964–52971 (2003).
Gavin, A. -C. et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415, 141–147 (2002).
Ho, Y. et al. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415, 180–183 (2002).
Li, S. et al. A map of the interactome network of the metazoan C. elegans. Science 303, 540–543 (2004).
Giot, L. et al. A protein interaction map of Drosophila melanogaster. Science 302, 1727–1736 (2003).
Colland, F. et al. Functional proteomic mapping of a human signaling pathway. Genome Res. 14, 1324–1332 (2004).
Bouwmeester, T. et al. A physical and functional map of the human TNF-α/NF-κB signal transduction pathway. Nature Cell Biol. 6, 97–105 (2004).
Dorn, G. et al. Specific inhibition of the rat ligand-gated ion channel P2X3 function via methoxyethoxy-modified phosphorothioated antisense oligonucleotides. Antisense Nucleic Acid Drug Dev. 11, 165–174 (2001).
Wahlestedt, C. et al. Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc. Natl Acad. Sci. USA 97, 5633–5638 (2000).
Good, L., Awasthi, S. K., Dryselius, R., Larsson, O. & Nielsen, P. E. Bactericidal antisense effects of peptide–PNA conjugates. Nature Biotechnol. 19, 360–364 (2001).
McCaffrey, A. P., Meuse, L., Karimi, M., Contag, C. H. & Kay, M. A. A potent and specific morpholino antisense inhibitor of hepatitis C translation in mice. Hepatology 38, 503–508 (2003).
Mercatante, D., Mohler, J. & Kole, R. Cellular response to an antisense-mediated shift of bcl-x mRNA splicing and antineoplastic agents. J. Biol. Chem. 277, 49374–49382 (2002).
Karras, J. G., Maier, M. A., Lu, T., Watt, A. & Manohran, M. P. Peptide nucleic acids are potent modulators of endogenous pre-mRNA splicing of the murine interleukin-5 receptor-α chain. Biochemistry 40, 7853–7859 (2001).
Hall, J. Unraveling the general properties of siRNAs: strength in numbers and lessons from the past. Nature Rev. Genet. 5, 552–227 (2004).
Kisielow, M., Kleiner, S., Magasawa, M., Faisal, A. & Nagamine, M. Isoform-specific knockdown and expression of adaptor protein shc A using small interfering RNA. Biochem. J. 363, 1–5 (2002).
Brummelkamp, T. R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002).
Rubinson, D. A. et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nature Genet. 33, 401–406 (2003).
Rushworth, S. A. et al. Inhibition of porcine DC40 expression and function by in vitro transfection with antisense oligonucleotides. Transplantation 73, 635–642 (2002).
Rijcken, E. et al. ICAM-1 and VCAM-1 antisense oligonucleotides attenuate in vivo leukocyte adherence and inflammation in rat inflammatory bowel disease. Gut 51, 529–535 (2002).
Hemmings-Mieszczak, M., Dorn, G., Natt, F., Hall, J. & Wishart, W. Independent combinatorial effect of antisense oligonucleotides and RNAi-mediated specific inhibition of the recombinant rat P2X3 receptor. Nucleic Acids Res. 31, 2117–2126 (2003).
Barclay, J. et al. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J. Neurosci. 22, 8139–8147 (2002).
Dorn, G. et al. siRNA relieves chronic neuropathic pain. Nucleic Acids Res. 32, e49 (2004). References 52 and 54 demonstrated the use of antisense and small interfering RNA, respectively, in animal models of disease.
McGraughty, S. et al. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br. J. Pharmacol. 140, 1381–1388 (2003).
Strausberg, R. L. et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc. Natl Acad. Sci. USA 99, 16899–16903 (2002). Describes the creation of the Mammalian Gene Collection, an important resource for functional genomics.
Walhout, A. J. et al. GATEWAY recombinational cloning: application to the cloning of large numbers of open reading frames or ORFeomes. Methods Enzymol. 328, 575–592 (2000).
Simpson, J. C., Wellenreuther, R., Poustka, A., Pepperkok, R. & Wiemann, S. Systematic subcellular localization of novel proteins identified by large scale cDNA sequencing. EMBO Rep. 3, 287–292 (2003).
Michiels, F. et al. Arrayed adenoviral expression libraries for functional screening. Nature Biotechnol. 20, 1154–1157 (2002).
Matsuda, A. et al. Large-scale identification and characterization of human genes that activate NF-κB and MAPK signaling pathways. Oncogene 22, 2307–2318 (2003).
Fiscella, M. et al. TIP, a T-cell factor identified using high-throughput screening increases survival in a graft-versus-host disease model. Nature Biotechnol. 21, 302–307 (2003).
Chen, C. et al. An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nature Biotechnol. 21, 294–301 (2003).
Iourgenko, V. et al. Identification of family of cAMP response element-binding protein co-activators by genome-scale functional analysis in mammalian cells. Proc. Natl Acad. Sci. USA 100, 12147–12152 (2003). Describes one of the first high-throughput genomic screens using overexpression from a full-length cDNA collection.
Chanda, S. K. et al. Genome-scale functional profiling of the mammalian AP-1 signaling pathway. Proc. Natl Acad. Sci. USA 100, 12153–12158 (2003).
Paddison, P. J. et al. A resource for large-scale RNA-interference-based screens in mammals. Nature 428, 427–431 (2004).
Berns, K. et al. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428, 431–437 (2004). References 65 and 66 demonstrated that large-scale RNA interference screens are of potential use for gene analysis and discovery.
Li, H. & Garza, D. in Model Organisms in Drug Discovery (eds Carroll, P. M. & Fitzgerald, K.) 81–117 (Wiley, Chichester, 2003).
Konsolaki, M. & Cohen, D. Targets for Alzheimer's disease: lessons learnt from flies. Drug Discov. Today 3, 64–70 (2004).
Langheinrich, U. Zebrafish: a new model on the pharmaceutical catwalk. Bioessays 25, 904–912 (2003).
Milan, D. J., Peterson, T. A., Ruskin, J. N., Peterson, R. T. & MacRae, C. A. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 107, 1355–1358 (2003).
Fermini, B. & Fossa, A. A. The impact of drug-induced QT interval prolongation on drug discovery and development. Nature Rev. Drug Discov. 2, 439–447 (2003).
Chan, J., Bayliss, P. E., Wood, J. M. & Roberts, T. M. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell 1, 257–267 (2002).
Farber, S. A. et al. Genetic analysis of digestive physiology using fluorescent phospholipids reporters. Science 292, 1385–1388 (2001).
Darland, T. & Dowling, J. E. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc. Natl Acad. Sci. USA 98, 11691–11696 (2001).
Lockwood, B., Bjerke, S., Kobayashi, K. & Guo, S. Acute effects of alcohol on larval zebrafish: a genetic systems for large-scale screening. Pharmaco. Biochem. Behav. 77, 647–654 (2004).
Peterson, R. T. et al. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nature Biotechnol. 22, 595–599 (2004).
Finelli, A., Kelkar, A., Song, H., Yang, H. & Konsolaki, M. A model for Alzheimer's Aβ42-induced toxicity in Drosophila melanogaster. Mol. Cell. Neurosci. 26, 365–375 (2004). Demonstrates the use of a genetically tractable model organism to screen for novel elements in disease-related pathways.
Acknowledgements
The authors would like to thank F. Steele, N. Nanguneri, Q. Ma, F. Buxton, J. van Oostrum, J. Hall, M. Labow, D. Garza, M. Konsolaki and F. Serluca for their contributions to this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R.K. and D.C. are employees of Novartis.
Glossary
- ORPHAN RECEPTOR
-
A receptor for which the ligand has not been identified.
- TWO-DIMENSIONAL GEL ELECTROPHORESIS
-
A commonly used method for fractionating proteins, in which proteins are first separated (first dimension) on a poly-acrylamide gel according to isoelectric point, then separated at a 90° angle (second dimension) on the basis of molecular mass.
- WATSON–CRICK BINDING
-
The binding of one strand of a nucleic acid with another strand through the pairing of complementary bases (A–T and G–C) to form a 'double-helix' structure.
Rights and permissions
About this article
Cite this article
Kramer, R., Cohen, D. Functional genomics to new drug targets. Nat Rev Drug Discov 3, 965–972 (2004). https://doi.org/10.1038/nrd1552
Issue Date:
DOI: https://doi.org/10.1038/nrd1552
This article is cited by
-
Sequence-based design of bioactive small molecules that target precursor microRNAs
Nature Chemical Biology (2014)
-
Chemical genetics strategies for identification of molecular targets
Phytochemistry Reviews (2013)
-
Innovations in studying in vivo cell behavior and pharmacology in complex tissues – microvascular endothelial cells in the spotlight
Cell and Tissue Research (2013)
-
Multiplexed mass cytometry profiling of cellular states perturbed by small-molecule regulators
Nature Biotechnology (2012)