Key Points
-
Heart failure (HF) is the leading cause of death in the Western world.
-
Abnormal intracellular Ca2+ handling is crucial to the pathogenesis of heart failure, and is an important contributor to a decrease in ventricular contractile function.
-
Hyperactivity of the sympathetic nervous system is important role in sustaining abnormal intracellular Ca2+ release.
-
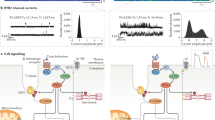
Protein kinase A (PKA) hyperphosphorylation of the cardiac ryanodine receptor (RyR2) leads to dissociation of the channel-stabilizing protein calstabin2, which is associated with diastolic Ca2+ leak and a reduction in cardiac contractility.
-
β-adrenoceptor blockers are one class of the few drugs that reduce mortality in CHF patients. Beta-blockers reverse PKA-hyperphosphorylation of RyR2 and increase calstabin2 binding, which might contribute to increased contractility in patients with CHF.
-
Specific inhibitors for G-protein-coupled receptor kinase (GRK) or protein kinase C (PKC-α) are promising therapeutic targets to normalize intracellular signalling in the failing heart.
-
New drugs designed to increase sarcoplasmic reticulum Ca2+ loading in CHF could potentially induce cardiac arrhythmias.
-
The 1,4-benzothiazepine JTV519 increases calstabin2 binding to RyR2 in failing hearts. By preventing intracellular Ca2+ leak, JTV519 increases cardiac contractility and decreases the propensity to cardiac arrhythmias in CHF.
Abstract
Congestive heart failure is the leading cause of death in the Western world. Abnormal intracellular calcium (Ca2+) handling is central to the pathogenesis of heart failure because it contributes to a decrease in ventricular contractile function. Chronic hyperactivity of the sympathetic nervous system causes increased phosphorylation of the ryanodine receptor intracellular Ca2+-release channel, a key Ca2+-handling protein in the heart, by protein kinase A. Alteration of the structure and function of ryanodine receptors contributes to defective intracellular Ca2+ handling and an increased propensity for cardiac arrhythmias in failing hearts. Novel therapeutic strategies are now being evaluated to specifically correct defective Ca2+-handling in heart failure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hunt, S. A. et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. J. Am. Coll. Cardiol. 38, 2101–2113 (2001).
Pieske, B., Maier, L. S., Bers, D. M. & Hasenfuss, G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ. Res. 85, 38–46 (1999).
Bers, D. M. Cardiac excitation–contraction coupling. Nature 415, 198–205 (2002).
Fill, M. & Copello, J. A. Ryanodine receptor calcium release channels. Physiol. Rev. 82, 893–922 (2002).
Wehrens, X. H. & Marks, A. R. Altered function and regulation of cardiac ryanodine receptors in cardiac disease. Trends Biochem. Sci. 28, 671–678 (2003).
Sipido, K. R., Carmeliet, E. & Van de Werf, F. T-type Ca2+ current as a trigger for Ca2+ release from the sarcoplasmic reticulum in guinea-pig ventricular myocytes. J. Physiol. 508, 439–451 (1998).
Sipido, K. R., Maes, M. & Van de Werf, F. Low efficiency of Ca2+ entry through the Na+-Ca2+ exchanger as trigger for Ca2+ release from the sarcoplasmic reticulum. A comparison between L-type Ca2+ current and reverse-mode Na+–Ca2+ exchange. Circ. Res. 81, 1034–1044 (1997).
Fabiato, A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 245, C1–C14 (1983). A landmark paper that provided the first description and characterization of the excitation–contraction coupling process in cardiac myocytes.
Koss, K. L., Grupp, I. L. & Kranias, E. G. The relative phospholamban and SERCA2 ratio: a critical determinant of myocardial contractility. Basic Res. Cardiol. 92 (Suppl. 1), 17–24 (1997).
Jones, L. R., Simmerman, H. K., Wilson, W. W., Gurd, F. R. & Wegener, A. D. Purification and characterization of phospholamban from canine cardiac sarcoplasmic reticulum. J. Biol. Chem. 260, 7721–7730 (1985).
Bers, D. M. & Bridge, J. H. Relaxation of rabbit ventricular muscle by Na–Ca exchange and sarcoplasmic reticulum calcium pump. Ryanodine and voltage sensitivity. Circ. Res. 65, 334–342 (1989).
Marx, S. O. et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101, 365–376 (2000). The first report on PKA-hyperphosphorylation of the cardiac ryanodine receptors (RyR2) as a cause of abnormal Ca2+ cycling and impaired contractility in human heart failure.
Wehrens, X. H., Lehnart, S. E., Reiken, S. R. & Marks, A. R. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ. Res. 94, e61–e70 (2004).
Braz, J. C. et al. PKC-α regulates cardiac contractility and propensity toward heart failure. Nature Med. 10, 248–254 (2004). A demonstration of the role of PKC-α as a nodal integrator of cardiac contractility by sensing intracellular Ca2+ and signal transduction events. Altered PKC-α signalling can profoundly affect the propensity toward heart failure.
Rockman, H. A., Koch, W. J. & Lefkowitz, R. J. Seven-transmembrane-spanning receptors and heart function. Nature 415, 206–212 (2002).
Gomez, A. M. et al. Defective excitation–contraction coupling in experimental cardiac hypertrophy and heart failure. Science 276, 800–806 (1997).
Dzhura, I., Wu, Y., Colbran, R. J., Balser, J. R. & Anderson, M. E. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nature Cell Biol. 2, 173–177 (2000).
DeSantiago, J., Maier, L. S. & Bers, D. M. Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban. J. Mol. Cell. Cardiol. 34, 975–984 (2002).
Maier, L. S. et al. Transgenic CaMKIIδC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ. Res. 92, 904–911 (2003).
Wu, Y., MacMillan, L. B., McNeill, R. B., Colbran, R. J. & Anderson, M. E. CaM kinase augments cardiac L-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. Am. J. Physiol. 276, H2168–H2178 (1999).
Napolitano, R., Vittone, L., Mundina, C., Chiappe de Cingolani, G. & Mattiazzi, A. Phosphorylation of phospholamban in the intact heart. A study on the physiological role of the Ca2+-calmodulin-dependent protein kinase system. J. Mol. Cell. Cardiol. 24, 387–396 (1992).
Hagemann, D. et al. Frequency-encoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J. Biol. Chem. 275, 22532–22536 (2000).
Hagemann, D. & Xiao, R. P. Dual site phospholamban phosphorylation and its physiological relevance in the heart. Trends Cardiovasc. Med. 12, 51–56 (2002).
Dempsey, E. C. et al. Protein kinase C isozymes and the regulation of diverse cell responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L429–L438 (2000).
Wang, J., Liu, X., Arneja, A. S. & Dhalla, N. S. Alterations in protein kinase A and protein kinase C levels in heart failure due to genetic cardiomyopathy. Can. J. Cardiol. 15, 683–690 (1999).
Sugden, P. H. & Bogoyevitch, M. A. Intracellular signalling through protein kinases in the heart. Cardiovasc. Res. 30, 478–492 (1995).
Pass, J. M. et al. Enhanced PKCβ II translocation and PKC-β II-RACK1 interactions in PKCε-induced heart failure: a role for RACK1. Am. J. Physiol. Heart Circ. Physiol. 281, H2500–H2510 (2001).
Tunwell, R. E. et al. The human cardiac muscle ryanodine receptor-calcium release channel: identification, primary structure and topological analysis. Biochem. J. 318, 477–487 (1996).
Otsu, K. et al. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J. Biol. Chem. 265, 13472–13483 (1990). A report of the molecular cloning of the cardiac ryanodine receptor from rabbit heart, showing that the RyR2 isoform contains almost 5,000 amino acids and has 67% sequence similarity to the skeletal muscle isoform RyR1.
Chu, A., Sumbilla, C., Inesi, G., Jay, S. D. & Campbell, K. P. Specific association of calmodulin-dependent protein kinase and related substrates with the junctional sarcoplasmic reticulum of skeletal muscle. Biochemistry 29, 5899–5905 (1990).
Brillantes, A.–M. B. et al. FKBP12 Optimizes function of the cloned expressed calcium release channel (ryanodine receptor). Biophys. J. 66, A19 (1994).
Marx, S. O. et al. Phosphorylation-dependent regulation of ryanodine receptors. A novel role for leucine/isoleucine zippers. J. Cell. Biol. 153, 699–708 (2001). Demonstration that the ryanodine receptor is a macromolecular complex, and that protein kinase A and protein phosphatases PP1 and PP2 are targeted to RyR2 via specific adaptor proteins through leucine/ isoleucine zipper motifs.
Currie, S., Loughrey, C. M., Craig, M. A. & Smith, G. L. Calcium/calmodulin-dependent protein kinase IIδ associates with the ryanodine receptor complex and regulates channel function in rabbit heart. Biochem. J. 377, 357–366 (2004).
Meyers, M. B. et al. Association of sorcin with the cardiac ryanodine receptor. J. Biol. Chem. 270, 26411–26418 (1995).
Zhang, L., Kelley, J., Schmeisser, G., Kobayashi, Y. M. & Jones, L. R. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 272, 23389–23397 (1997). Study establishing the binding of several proteins in the sarcoplasmic reticulum to the luminal side of the ryanodine receptor.
Flucher, B. E. et al. Triad formation: organization and function of the sarcoplasmic reticulum calcium release channel and triadin in normal and dysgenic muscle in vitro. J. Cell. Biol. 123, 1161–1174 (1993).
Collins, J., Tarcsafalvi, A. & Ikemoto, N. Identification of a region of calsequestrin that binds to the junctional face membrane of sarcoplasmic reticulum. Biochem. Biophys. Res. Commun. 167, 189–193 (1990).
Viatchenko-Karpinski, S. et al. Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin. Circ. Res. 94, 471–477 (2004).
Wehrens, X. H. et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113, 829–840 (2003).
Marx, S. O., Ondrias, K. & Marks, A. R. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors). Science 281, 818–821 (1998).
Gaburjakova, M. et al. FKBP12 binding modulates ryanodine receptor channel gating. J. Biol. Chem. 276, 16931–16935 (2001).
Hain, J., Onoue, H., Mayrleitner, M., Fleischer, S. & Schindler, H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. J. Biol. Chem. 270, 2074–2081 (1995).
Valdivia, H. H., Kaplan, J. H., Ellis-Davies, G. C. & Lederer, W. J. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science 267, 1997–2000 (1995).
Lokuta, A. J., Rogers, T. B., Lederer, W. J. & Valdivia, H. H. Modulation of cardiac ryanodine receptors of swine and rabbit by a phosphorylation-dephosphorylation mechanism. J. Phys. 487, 609–622 (1995).
Sonnleitner, A., Fleischer, S. & Schindler, H. Gating of the skeletal calcium release channel by ATP is inhibited by protein phosphatase 1 but not by Mg2+. Cell Calcium 21, 283–290 (1997).
Terentyev, D., Viatchenko-Karpinski, S., Gyorke, I., Terentyeva, R. & Gyorke, S. Protein phosphatases decrease sarcoplasmic reticulum calcium content by stimulating calcium release in cardiac myocytes. J. Physiol. 552, 109–118 (2003).
Beuckelmann, D., Nabauer, M. & Erdmann, E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation 85, 1046–1055 (1992). An important paper providing more insight into abnormalities in Ca2+ cycling in myocytes isolated from patients with heart failure.
Beuckelmann, D. J., Nabauer, M., Kruger, C. & Erdmann, E. Altered diastolic Ca handling in human ventricular myocytes from patients with terminal heart failure. Am. Heart J. 129, 684–689 (1995).
Kluger, J., Cody, R. J. & Laragh, J. H. The contributions of sympathetic tone and the renin–angiotensin system to severe chronic congestive heart failure: response to specific inhibitors (prazosin and captopril). Am. J. Cardiol. 49, 1667–1674 (1982).
Cohn, J. N. et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 311, 819–823 (1984).
Bristow, M. R. et al. Reduced β1 receptor messenger RNA abundance in the failing human heart. J. Clin. Invest. 92, 2737–2745 (1993).
Daaka, Y., Luttrell, L. M. & Lefkowitz, R. J. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 390, 88–91 (1997).
Ungerer, M., Bohm, M., Elce, J. S., Erdmann, E. & Lohse, M. J. Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in failing human heart. Circulation 87, 454–463 (1993).
Chen, X. et al. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ. Res. 91, 517–524 (2002).
Wei, S. K. et al. Protein kinase A hyperphosphorylation increases basal current but decreases β-adrenergic responsiveness of the sarcolemmal Na+–Ca2+ exchanger in failing pig myocytes. Circ. Res. 92, 897–903 (2003).
Brillantes, A., Allen, P. & Marks, A. Molecular cloning of the human cardiac calcium release channel cDNA: expression studies in end-stage human heart Failure. Circulation 84 (Suppl. II), 442 (1991).
Dipla, K., Mattiello, J. A., Margulies, K. B., Jeevanandam, V. & Houser, S. R. The sarcoplasmic reticulum and the Na+/Ca2+ exchanger both contribute to the Ca2+ transient of failing human ventricular myocytes. Circ. Res. 84, 435–444 (1999).
Lowes, B. D. et al. Myocardial gene expression in dilated cardiomyopathy treated with β-blocking agents. N. Engl. J. Med. 346, 1357–1365 (2002).
Reiken, S. et al. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts. Role of phosphatases and response to isoproterenol. J. Biol. Chem. 278, 444–453 (2003).
Yano, M. et al. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca2+ leak through ryanodine receptor in heart failure. Circulation 102, 2131–2136 (2000).
MERIT-HF. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353, 2001–2007 (1999).
Packer, M. et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U. S. Carvedilol Heart Failure Study Group. N. Engl. J. Med. 334, 1349–1355 (1996). Landmark randomized controlled clinical trail showing that β-adrenoceptor blockers decrease mortality in patients with heart failure.
CIBIS-II. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 353, 9–13 (1999).
Reiken, S. et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation 107, 2459–2466 (2003).
Reiken, S. et al. β-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation 104, 2843–2848 (2001).
Doi, M. et al. Propranolol prevents the development of heart failure by restoring FKBP12.6-mediated stabilization of ryanodine receptor. Circulation 105, 1374–1379 (2002).
Masson, S., Chimenti, S. & Salio, M. CHF-1024, a DA2/α2 agonist, blunts norepinephrine excretion and cardiac fibrosis in pressure overload. Cardiovasc. Drug Ther. 15, 131–138 (2001).
Bayes, M., Rabasseda, X. & Prous, J. R. Gateways to clinical trials. Methods Find. Exp. Clin. Pharmacol. 25, 565–597 (2003).
Freedman, N. J. et al. Phosphorylation and desensitization of the human β1-adrenergic receptor. Involvement of G-protein-coupled receptor kinases and cAMP-dependent protein kinase. J. Biol. Chem. 270, 17953–17961 (1995).
Harding, V. B., Jones, L. R., Lefkowitz, R. J., Koch, W. J. & Rockman, H. A. Cardiac β-ARK1 inhibition prolongs survival and augments beta-blocker therapy in a mouse model of severe heart failure. Proc. Natl Acad. Sci. USA 98, 5809–5814 (2001).
Rockman, H. A. et al. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc. Natl Acad. Sci. USA 95, 7000–7005 (1998). Experimental study demonstrating the important role of β-adrenoceptor kinase inhibitors as modifiers of β-adrenoceptor signalling in the development of heart failure.
Gullestad, L. et al. Effect of metoprolol CR/XL on exercise tolerance in chronic heart failure — a substudy to the MERIT-HF trial. Eur. J. Heart Fail. 3, 463–468 (2001).
White, M. et al. Role of β-adrenergic receptor downregulation in the peak exercise response in patients with heart failure due to idiopathic dilated cardiomyopathy. Am. J. Cardiol. 76, 1271–1276 (1995).
Most, P. & Koch, W. J. Sealing the leak, healing the heart. Nature Med. 9, 993–934 (2003).
Wehrens, X. H. et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 304, 292–296 (2004).
Kohno, M. et al. A new cardioprotective agent, JTV519, improves defective channel gating of ryanodine receptor in heart failure. Am. J. Physiol. Heart Circ. Physiol. 284, H1035–H1042 (2003).
Yano, M. et al. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation 107, 477–484 (2003).
Hajjar, R. J. et al. Modulation of ventricular function through gene transfer in vivo. Proc. Natl Acad. Sci. USA 95, 5251–5256 (1998).
Minamisawa, S. et al. Chronic phospholamban–sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell 99, 313–322 (1999).
Meyer, M. & Dillmann, W. H. Sarcoplasmic reticulum Ca2+-ATPase overexpression by adenovirus mediated gene transfer and in transgenic mice. Cardiovasc. Res. 37, 360–366 (1998).
Ohizumi, Y., Sasaki, N. & Shibusawa, K. Stimulation of sarcoplasmic reticulum Ca2+-ATPase by gingerol analogous. Biol. Pharm. Bull. 19, 1377–1379 (1996).
Berrebi-Bertrand, I., Lahouratete, P. & Lahouratete, V. Mechanisms of action of sarcoplasmic reticulum calcium-uptake activators: discrimination between sarcoplasmic reticulum Ca2+-ATPase and phospholamban interaction. Eur. J. Biochem. 247, 801–809 (1997).
Volders, P. G. et al. Similarities between early and delayed afterdepolarizations induced by isoproterenol in canine ventricular myocytes. Cardiovasc. Res. 34, 348–359 (1997).
Hoshijima, M. et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nature Med. 8, 864–871 (2002).
Iwanaga, Y. et al. Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J. Clin. Invest. 113, 727–736 (2004).
del Monte, F., Harding, S. E., Dec, G. W., Gwathmey, J. K. & Hajjar, R. J. Targeting phospholamban by gene transfer in human heart failure. Circulation 105, 904–907 (2002).
Haghighi, K. et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J. Clin. Invest. 111, 869–876 (2003).
Bowling, N. et al. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation 99, 384–391 (1999).
Wang, J., Liu, X., Sentex, E., Takeda, N. & Dhalla, N. S. Increased expression of protein kinase C isoforms in heart failure due to myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 284, H2277–H2287 (2003).
Hahn, H. S. et al. Protein kinase Cα negatively regulates systolic and diastolic function in pathological hypertrophy. Circ. Res. 93, 1111–1119 (2003).
Vlahos, C. J., McDowell, S. A. & Clerk, A. Kinases as therapeutic targets for heart failure. Nature Rev. Drug Discov. 2, 99–113 (2003).
Mattiello, J. A., Margulies, K. B., Jeevanandam, V. & Houser, S. R. Contribution of reverse-mode sodium–calcium exchange to contractions in failing human left ventricular myocytes. Cardiovasc. Res. 37, 424–431 (1998).
Iwamoto, T., Watano, T. & Shigekawa, M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J. Biol. Chem. 271, 22391–22397 (1996).
Hobai, I. A. & O'Rourke, B. Enhanced Ca2+-activated Na+–Ca2+ exchange activity in canine pacing-induced heart failure. Circ. Res. 87, 690–698 (2000).
Shigekawa, M. & Iwamoto, T. Cardiac Na+–Ca2+ exchange: molecular and pharmacological aspects. Circ. Res. 88, 864–876 (2001).
Prestle, J. et al. Overexpression of FK506-binding protein FKBP12.6 in cardiomyocytes reduces ryanodine receptor-mediated Ca2+ leak from the sarcoplasmic reticulum and increases contractility. Circ. Res. 88, 188–194 (2001).
Miyamoto, M. I. et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc. Natl Acad. Sci. USA 97, 793–798 (2000).
Ennis, I. L., Li, R. A., Murphy, A. M., Marban, E. & Nuss, H. B. Dual gene therapy with SERCA1 and Kir2.1 abbreviates excitation without suppressing contractility. J. Clin. Invest. 109, 393–400 (2002).
Ji, Y., Loukianov, E., Loukianova, T., Jones, L. R. & Periasamy, M. SERCA1a can functionally substitute for SERCA2a in the heart. Am. J. Physiol. 276, H89–H97 (1999).
Magee, W. P. et al. Differing cardioprotective efficacy of the Na+/Ca2+ exchanger inhibitors SEA0400 and KB-R7943. Am. J. Physiol. Heart Circ. Physiol. 284, H903–H910 (2003).
Wehrens, X. H., Vos, M. A., Doevendans, P. A. & Wellens, H. J. Novel insights in the congenital long QT syndrome. Ann. Intern. Med. 137, 981–992 (2002).
Marks, A. R., Priori, S., Memmi, M., Kontula, K. & Laitinen, P. J. Involvement of the cardiac ryanodine receptor/calcium release channel in catecholaminergic polymorphic ventricular tachycardia. J. Cell. Physiol. 190, 1–6 (2002).
Leenhardt, A. et al. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation 91, 1512–1519 (1995).
Laitinen, P. J. et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 103, 485–490 (2001).
Priori, S. G. et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103, 196–200 (2001).
Fisher, J. D., Krikler, D. & Hallidie-Smith, K. A. Familial polymorphic ventricular arrhythmias: a quarter century of successful medical treatment based on serial exercise-pharmacologic testing. J. Am. Coll. Cardiol. 34, 2015–2022 (1999).
Swan, H. et al. Arrhythmic disorder mapped to chromosome 1q42–q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J. Am. Coll. Cardiol. 34, 2035–2042 (1999).
George, C. H., Higgs, G. V. & Lai, F. A. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ. Res. 93, 531–540 (2003).
Jiang, D., Xiao, B., Zhang, L. & Chen, S. R. Enhanced basal activity of a cardiac Ca2+ release channel (ryanodine receptor) mutant associated with ventricular tachycardia and sudden death. Circ. Res. 91, 218–225 (2002).
Kaneko, N. New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug Dev. Res. 33, 429–438 (1994).
Kaneko, N., Ago, H., Matsuda, R., Inagaki, E. & Miyano, M. Crystal structure of annexin V with its ligand K-201 as a calcium channel activity inhibitor. J. Mol. Biol. 274, 16–20 (1997).
Kawabata, H., Ryomoto, T. & Ishikawa, K. Effect of a novel cardioprotective agent, JTV-519, on metabolism, contraction and relaxation in the ischemia-reperfused rabbit heart. Jpn Circ. J. 64, 772–776 (2000).
Inagaki, K., Kihara, Y., Izumi, T. & Sasayama, S. The cardioprotective effects of a new 1,4-benzothiazepine derivative, JTV519, on ischemia/reperfusion-induced Ca2+ overload in isolated rat hearts. Cardiovasc. Drugs Ther. 14, 489–495 (2000).
Inagaki, K. et al. Anti-ischemic effect of a novel cardioprotective agent, JTV519, is mediated through specific activation of delta-isoform of protein kinase C in rat ventricular myocardium. Circulation 101, 797–804 (2000).
Ito, K. et al. JTV-519, a novel cardioprotective agent, improves the contractile recovery after ischaemia-reperfusion in coronary perfused guinea-pig ventricular muscles. Br. J. Pharmacol. 130, 767–76 (2000).
Nakaya, H., Furusawa, Y., Ogura, T., Tamagawa, M. & Uemura, H. Inhibitory effects of JTV-519, a novel cardioprotective drug, on potassium currents and experimental atrial fibrillation in guinea-pig hearts. Br. J. Pharmacol. 131, 1363–1372 (2000).
Kumagai, K., Nakashima, H., Gondo, N. & Saku, K. Antiarrhythmic effects of JTV-519, a novel cardioprotective drug, on atrial fibrillation/flutter in a canine sterile pericarditis model. J. Cardiovasc. Electrophysiol. 14, 880–884 (2003).
Schlotthauer, K. & Bers, D. M. Sarcoplasmic reticulum Ca2+ release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ. Res. 87, 774–780 (2000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors are employed by Columbia University, which has filed patents based on some of the authors' findings described in this article.
Related links
Related links
DATABASES
Entrez Gene
FURTHER INFORMATION
Glossary
- MYOCYTES
-
Muscle cells that contract rhythmically in the heart.
- SYSTOLE
-
The phase of the cardiac cycle during which the ventricles contract.
- DIASTOLE
-
The phase of the cardiac cycle during which the ventricles are relaxed.
- TRANSVERSE (T) TUBULE
-
An invagination of the plasma membrane that contains ion channels and ion transporters that are in a close spatial relationship with ion channels on the sarcoplasmic reticulum (to enable efficient excitation–contraction coupling).
- CATECHOLAMINES
-
Hormones (for example, adrenaline and noradrenaline) that affect the sympathetic nervous system, produced in the medulla of the adrenal gland. Catecholamines are derivatives of the steroid catechol, which is derived from the amino acid tyrosine.
- EC COUPLING GAIN
-
The ability of Ca2+ influx through voltage-gated L-type Ca2+ channels to trigger Ca2+ release from the sarcoplasmic reticulum.
- FIGHT-OR-FLIGHT RESPONSE
-
An evolutionarily conserved mechanism which allows for the rapid enhancement of cardiac contractility and cardiac output during exercise or sudden stress. This stress response is mediated by the activation of the sympathetic nervous system, which leads to phosphorylation of an array of intracellular proteins in the heart, including ryanodine receptor-2, by protein kinase A.
- CA2+-RELEASE UNITS
-
Structures containing two proteins essential to EC coupling: the L-type Ca2+ channels (LTCC) on the plasma membrane and the ryanodine receptors (RyR2) on the sarcoplasmic reticulum. The LTCC–RyR2 complexes are organized in to lattices which allow a large population of receptors to be simultaneously switched on, or off, by a very small change in ligand concentration.
Rights and permissions
About this article
Cite this article
Wehrens, X., Marks, A. Novel therapeutic approaches for heart failure by normalizing calcium cycling. Nat Rev Drug Discov 3, 565–574 (2004). https://doi.org/10.1038/nrd1440
Issue Date:
DOI: https://doi.org/10.1038/nrd1440
This article is cited by
-
Mechanisms underlying pathological Ca2+ handling in diseases of the heart
Pflügers Archiv - European Journal of Physiology (2021)
-
Convallatoxin enhance the ligand-induced mu-opioid receptor endocytosis and attenuate morphine antinociceptive tolerance in mice
Scientific Reports (2019)
-
Acylated Ghrelin Protects the Hearts of Rats from Doxorubicin-Induced Fas/FasL Apoptosis by Stimulating SERCA2a Mediated by Activation of PKA and Akt
Cardiovascular Toxicology (2019)
-
17β-Estradiol and/or estrogen receptor alpha blocks isoproterenol-induced calcium accumulation and hypertrophy via GSK3β/PP2A/NFAT3/ANP pathway
Molecular and Cellular Biochemistry (2017)
-
Effects of thoracic epidural analgesia on plasma cAMP and cGMP levels in patients with heart failure
Journal of Cardiothoracic Surgery (2013)