Key Points
-
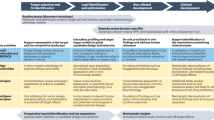
The aim of a first study of a new drug in humans is to explore in a safe and ethical manner the dose and exposure range that is well tolerated, and, if possible, to identify any dose-limiting adverse events. Achievement of these aims represents a major leap from the laboratory bench to humans. It requires a substantial body of information characterizing the test substance, which can only be derived from animal studies.
-
Despite these activites being regulated by many national and international guidelines, the approach to preclinical studies remains largely empirical. There is a paucity of evidence about the performance of the widely employed preclinical tests in prediction of toxicity in humans. There is a need for much better performance data in this area.
-
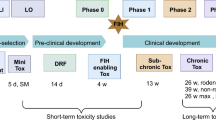
The available limited, retrospective evidence indicates that the conventional approach using experimental pharmacology alongside toxicity studies of one month's duration reasonably predicts adverse events in the first human studies. The conventional methods identify more than 90% of toxicities that can be detected in animals.
-
If toxicity studies are shorter than one month, there is a risk of certain organ toxicities being overlooked. However, single studies seem to have the capacity to detect many of the most important potential adverse effects.
-
Data obtained from dog studies are frequently better predictors than data from rodent experiments.
-
Although uncommon, serious idiosyncratic drug reactions involving skin, liver and haemopoiesis — which conventional animal studies usually fail to predict — are major problems in drug development.
Abstract
The need for careful testing of new drugs in animal models before study in humans has been recognised by physicians since the First World War. Now, first human studies on new drugs are subject to detailed government guidelines, which in the European Union are presently being reinforced through the wide-ranging Clinical Trials Directive. However, despite their long history and widespread application, these guidelines are empirical and have been formulated with a paucity of critical scientific evidence. Here, we review the principles and the available, albeit limited, evidence that support the design and conduct of preclinical studies in a way that permits effective and safe first-dose studies of potential new medicines in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Editorial. War chemistry in the alleviation of suffering. J. Ind. Eng. Chem. 10, 673–674 (1918).
Leake, C. D. The pharmacologic evaluation of new drugs. JAMA 93, 1632–1634 (1929). Report of an early symposium on the testing of new drugs, which outlines some of the key principles for preclinical and clinical evaluation.
Geiling, E. M. K. & Cannon, P. R. Pathologic effects of elixir of sulphanilamide (diethylene glycol) poisoning. JAMA 111, 919–926 (1938). Detailed report on the effects and cause of death of patients treated with the elixir of sulphanilamide, and the principles that should be adopted for the study of new drugs.
Shuster, E. Fifty years later: the significance of the Nuremberg Code. N. Engl. J. Med. 337, 1436–1440 (1997).
Barnes, J. M. Are official recommendations for the testing of drugs for toxicity dangerous? Proc. Eur. Soc. Study Drug Toxicity 2, 57–63 (1963).
Committee for Proprietary Medicinal Products. Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. (The European Agency for the Evaluation of Medicinal Products, London, 2001).
Schuster, E. The Nuremberg Code: Hippocratic ethics and human rights. Lancet 351, 974–977 (1998).
World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 277, 925–926 (1997).
Lawson, D. H. Adverse drug reactions. Human Toxicol. 4, 122–126 (1985).
Igarashi, T., Nakane, S. & Kitagawa, T. Predictability of clinical adverse reactions of drugs by general pharmacology studies. J. Toxicol. Sci. 20, 77–92 (1995).
Fletcher, A. P. Drug safety tests and subsequent clinical experience. J. Roy. Soc. Med. 71, 693–696 (1978).
Olson, H. et al. Concordance of the toxicity of pharmaceuticals in humans and animals. Regul. Toxicol. Pharmacol. 32, 56–67 (2000). A review of multinational pharmaceutical data and the outcome of a workshop which compared adverse effects of a large series of novel drugs in humans with toxicity in animals.
Japanese Pharmaceutical Manufacturers Association. Literature survey on the correlation between animal toxicity findings and clinical adverse effects. (Japanese Pharmaceutical Manufacturers Association, Tokyo, 1994).
Prentis, R. A. & Walker, S. R. Trends in the development of new medicines by UK-owned pharmaceutical companies (1964–1980). Br. J. Clin. Pharmacol. 21, 437–443 (1986).
Lumley, C. In Animal Toxicity Studies: Their Relevance for Man (ed. Walker, S. R.) 49–56 (Quay, Lancaster, 1990).
Schein, P. S. et al. The evaluation of anticancer drugs in dogs and monkeys for the prediction of qualitative toxicities in man. Clin. Pharmacol. Ther. 11, 3–40 (1970). Critical evaluation of the qualitative toxicity of anticancer drugs in dogs and monkeys compared with humans.
Owens, A. H. Predicting anticancer drug effects in man from laboratory animal studies. J. Chron. Dis. 15, 223–228 (1962).
Freireich, E. J., Gehen, E. A., Rall, D. P., Schmidt, L. H. & Skipper, H. E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. Reports 50, 219–244 (1966). Highly cited article that provides a quantitative comparison of the toxicity of anticancer drugs in several animal species and humans.
Committee for Proprietary Medicinal Products. Points to consider in the assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products. (The European Agency for the Evaluation of Medicinal Products, London, 1997).
Crumb, W. & Cavero, I. QT interval prolongation by non-cardiovascular drugs: issues and solutions for novel drug development. Pharm. Sci. Technol. Today 2, 270–280 (1999).
De Ponti, F., Poluzzi, E. & Montanaro, N. QT-interval prolongation by non-cardiac drugs: lessons to be learned from recent experience. Eur. J. Clin. Pharmacol. 56, 1–18 (2000).
Fermini, B. & Fossa, A. A. The impact of drug-induced QT interval prolongation on drug discovery and development. Nature Rev. Drug Discov. 2, 439–447 (2003).
Davis, A. S. The preclinical assessment of QT interval prolongation: a comparison of in vitro and in vivo methods. Human Exp. Toxicol. 17, 677–680 (1998).
Nemery, B., Dinsdale, D. & Verschoyle, R. D. Detecting and evaluating chemical-induced lung damage in experimental animals. Bull. Eur. Physiopathol. Respir . 23, 501–528 (1987).
Dressman, J. B. Comparison of canine and human gastrointestinal physiology. Pharmacol. Res. 3, 123–131 (1986).
Lee, W. M. Drug-induced hepatotoxicity. N. Engl. J. Med. 349, 474–485 (2003).
Amacher, D. E. Serum transaminase elevations as indicators of hepatic injury following administration of drugs. Regul. Toxicol. Pharmacol. 27, 119–130 (1998).
Hayes, A. W. et al. Correlation of human hepatotoxicants with hepatic damage in animals. Fund. Appl. Toxicol. 2, 55–66 (1982).
Schwartz, S., Raskin, P., Fonseca, V. & Graveline, J. F. Effect of troglitazone in insulin-treated patients with type II diabetes mellitus. N. Engl. J. Med. 338, 861–866 (1998).
Mok, C. C., Lau, C. S. & Wong, R. W. S. Risk factors for ovarian failure in patients with systemic lupus erythematosus receiving cyclophosphamide therapy. Arthritis Rheum. 41, 831–837 (1998).
Takayama, S., Akaike, M., Kawashima, K., Takahashi, M. & Kurokawa, Y. A collaborative study in Japan on optimal treatment period and parameters for detection of male fertility disorders induced by drugs in rats. Regul. Toxicol. Pharmacol. 14, 266–292 (1984).
Ribelin, W. E. The effects of drugs and chemicals upon the structure of the adrenal gland. Fund. Appl. Toxicol. 4, 105–119 (1984).
Theus, R. & Zbinden, G. Toxicological assessment of the hemostatic system, regulatory requirements, and industry practice. Regul. Toxicol. Pharmacol. 4, 74–95 (1984).
Dayan, A. D. et al. Report of a validation study of assessment of direct immunotoxicity in the rat. Toxicology 125, 183–201 (1998).
Dean, J. H. Issues with introducing new immunotoxicology methods into the safety assessment of pharmaceuticals. Toxicology 119, 95–101 (1997).
Dean, J. H., Hinks, J. R. & Remander, B. Immunotoxicology assessment in the pharmaceutical industry. Toxicol. Lett. 102–103, 247–255 (1998).
Cavagnaro, J. A. Preclinical safety evaluation of biotechnology-derived pharmaceuticals. Nature Rev. Drug Discov. 1, 469–475 (2002).
Lichfield, J. T. Forecasting drug effects in man from studies in laboratory animals. JAMA 177, 104–108 (1961).
Steiness, E., Rasmussen, F., Svendsen, O. & Nielsen, P. A comparative study of serum creatine phosphokinase (CPK) activity in rabbits, pigs and humans after intramuscular injection of local damaging drugs. Acta Pharmacol. Toxicol. 42, 357–364 (1978).
Clive, D. Mutagenicity tests in drug development: interpretation and significance of test results. Regul. Toxicol. Pharmacol. 5, 79–100 (1985).
Benigni, R. & Zito, R. Designing safer drugs: (Q)SAR-based identification of mutagens and carcinogens. Curr. Topics Med. Chem. 3, 1289–1300 (2003).
Monro, A. & Mehta, D. Are single-dose toxicology studies in animals adequate to support single dose of a new drug in humans? Clin. Pharmacol. Ther. 59, 258–264 (1996). Provides the key arguments for testing new drugs in humans based on single-dose toxicity studies in animals.
Choudary, J. et al. Response to Monro and Mehta proposal for use of single-dose toxicity studies to support single-dose studies of new drugs in humans. Clin. Pharmacol. Ther. 59, 265–267 (1996).
Rozman, K. K. & Doull, J. The role of time as a quantifiable variable of toxicity and the experimental conditions when Haber's c x t product can be observed: implications for therapeutics. J. Pharmacol. Exp. Ther. 296, 663–668 (2001).
Combes, R. D. et al. Early microdose drug studies in human volunteers can minimise animal testing. Proceedings of a workshop organised by volunteers in research and testing. Eur. J. Pharm. Sci. 19, 1–11 (2003).
Lappin, G. & Garner, R. C. Big physics, small doses: the use of AMS and PET in human microdosing of development drugs. Nature Rev. Drug Discov. 2, 233–240 (2003).
Committee for Proprietary Medicinal Products. Position paper on non-clinical safety studies to support clinical trials with a single microdose. (The European Agency for the Evaluation of Medicinal Products, London, 2003).
Calabrese, E. J. & Baldwin, L. A. Toxicology rethinks its central belief. Nature 421, 691–692 (2003).
Monro, A. M. The role of metabolism studies in drug safety evaluation. Drug Dev. Comm. 2, 377–396 (1976).
Jorkasky, D. K. What does the clinician want to know from the toxicologist? Toxicol. Lett. 102–103, 539–543 (1998).
Grieshaber, C. K. & Marsoni, S. Relation of preclinical toxicology to findings in early clinical trials. Cancer Treat. Rep. 70, 65–72 (1986).
Greaves, P. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation (Elsevier, Amsterdam, 2000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Drs Greaves and Eve presently act as consultants for Marix Drug Development Ltd. Dr Greaves receives a pension from AstraZeneca, and has recently consulted for Shire Pharmaceutical Development Ltd, Experimental Pathology Laboratories Inc. and Aventis.
Glossary
- SULPHANILAMIDE
-
An old antimicrobial drug that is still occasionally used therapeutically.
- GOOD LABORATORY PRACTICE
-
(GLP). This defines a set of rules and criteria for the organizational processes and the conditions under which preclinical safety studies are planned, performed, monitored, recorded, reported and archived.
- GOOD MANUFACTURING PRACTICE
-
(GMP). This defines an assurance process that is similar to GLP. It ensures that products are consistently manufactured and controlled to the quality standards that are appropriate to their intended use.
- NECROPSY
-
A detailed post-mortem examination. Also referred to as autopsy.
- QT INTERVAL PROLONGATION
-
Increase in the total time of ventricular polarization as measured from the onset of the Q wave to the end of the T wave on the electrocardiogram of the heart.
- IDIOSYNCRATIC RESPONSES
-
Infrequent adverse responses to drugs that differ from predictable, dose-dependent toxicities. They are characterized by a variable delay or latency period and might cause severe injury and death.
- STEATOSIS
-
A cellular change characterized by an increase in lipid, usually seen as cytoplasmic droplets.
Rights and permissions
About this article
Cite this article
Greaves, P., Williams, A. & Eve, M. First dose of potential new medicines to humans: how animals help. Nat Rev Drug Discov 3, 226–236 (2004). https://doi.org/10.1038/nrd1329
Issue Date:
DOI: https://doi.org/10.1038/nrd1329
This article is cited by
-
Toxicogenomic module associations with pathogenesis: a network-based approach to understanding drug toxicity
The Pharmacogenomics Journal (2018)
-
Innovative organotypic in vitro models for safety assessment: aligning with regulatory requirements and understanding models of the heart, skin, and liver as paradigms
Archives of Toxicology (2018)