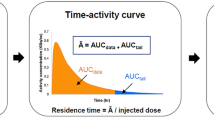
Abstract
The process of early clinical drug development has changed little over the past 20 years despite an up to 40% failure rate associated with inappropriate drug metabolism and pharmacokinetics of candidate molecules. A new method of obtaining human metabolism data known as microdosing has been developed which will permit smarter candidate selection by taking investigational drugs into humans earlier. Microdosing depends on the availability of two ultrasensitive 'big-physics' techniques: positron emission tomography (PET) can provide pharmacodynamic information, whereas accelerator mass spectrometry (AMS) provides pharmacokinetic information. Microdosing allows safer human studies as well as reducing the use of animals in preclinical toxicology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tufts Center for the Study of Drug Development. [online] (cited 03.02.2003) <http://csdd.tufts.edu/NewsEvents> (2002).
Lowest number of launches for 10 years! CMR International Report 20, 4–5 (2002).
New Drug Approval Reports [online], (cited 01.02.2003) <http://www.fda.gov/cder/rdmt/> (2003).
Bolten, B. M. and DeGregorio, T. Trends in development cycles. Nature Rev. Drug Discov. 1, 335–336 (2002).
Dimasi, J. A. Risks in new drug development: approval success rates for investigational drugs. Clin. Pharmacol. Ther. 69, 297–307 (2001).
Prentis, R. A., Lis, Y. & Walker, S. R. Pharmaceutical innovation by the seven UK-owned pharmaceutical companies (1964–1985). Br. J. Clin. Pharmacol. 25, 387–396 (1988).
Bajorath, J. Integration of virtual and high-throughput screening. Nature Rev. Drug Discov. 1, 882–894 (2002).
Blundell, T. L., Jhoti, H & Abell, C. High-throughput crystallography for lead discovery in drug design. Nature Rev Drug Discov. 1, 45–54 (2002).
Renaud, J. P. et al. Recombinant yeast in drug metabolism. Toxicology 82, 39–52 (1993).
Bachmann, K. A. & Ghosh, R. The use of in vitro methods to predict in vivo pharmacokinetics and drug interactions. Curr. Drug Metab. 3, 299–314 (2001).
Testino, S. A. and Patonay, G. High-throughput inhibition screening of major human cytochrome P450 enzymes using an in vitro cocktail and liquid chromatography tandem mass spectroscopy. J. Pharm. Biomed. Anal. 30, 1459–1467 (2003).
Mahmood, I. Interspecies scaling: predicting volumes, mean residence time and elimination half-life. Some considerations. J. Pharm. Pharmacol. 50, 493–499 (1998).
Jang, G. R., Harris, R. Z. & Lau, D. T. Pharmacokinetics and its role in small molecule drug discovery research. Med. Res. Rev. 21, 382–396 (2001).
Lesko, L. J., Rowland, M., Peck, C. C. & Blaschke, T. F. Optimizing the science of drug development: opportunities for better candidate selection and accelerated evaluation in humans. Eur. J. Pharm. Sci. 10, iv–xiv (2000).
Mahmood, I. Allometric issues in drug development. J. Pharm. Sci. 88, 1101–1106 (1999).
Turteltaub, K. W. & Vogel, J. S. Bioanalytical applications of accelerator mass spectrometry for pharmaceutical research. Curr. Pharm. Des. 6, 991–1007 (2000).
Aboagye, E. O., Price, P. M. & Jones, T. M. In vivo pharmacokinetics and pharmacodynamics in drug development using positron-emission tomography. Drug Discov. Today 6, 293–302 (2001).
Turteltaub, K. W. et al. Accelerator mass spectrometry in biomedical dosimetry: relationship between low exposure and covalent binding of heterocyclic amine carcinogens to DNA. Proc. Natl Acad. Sci. USA 87, 5288–5292 (1990).
The European Agency for the Evaluation of Medical Products Evaluation of Medicines for Human Use. Position paper on the non-clinical safety studies to support clinical trials with a single low dose of a compound. CPMP/SWP/2599/02 draft [online], (cited 01.02.2003) <http://www.emea.eu.int/pdfs/human/swp/259902en.pdf> (2002).
IEH testing requirements under the EC White Paper 'Strategy for a future chemicals policy'. (Institute of Environment and Health, Leicester, UK, 2001).
IEH testing requirements under the EC White Paper 'Strategy for a future chemicals policy – an update' (Draft). (Institute of Environment and Health, Leicester, UK, 2001).
Mathieu, M. A closer look at screening INDs. Applied Clinical Trials, April, 1–2 (2001).
Young, G., Ellis, W., Ayrton, J., Hussey, E. and Adamkiewicz, B. Accelerator mass spectrometry (AMS) recent experience of its use in a clinical study and the potential future of the technique. Xenobiotica 31, 619–632 (2001).
Williams, P. et al. Accelerator mass spectrometry and microdosing to evaluate the clinical pharmacokinetics of a new agent. Clin. Pharmacol. Ther. 71, 75 (2002).
Hartvig, P., Bergström, M. and Långström, B. Use of positron emission tomography in analysing receptor function in vivo. Toxicol. Lett. 120, 243–251 (2001).
Cherry, S. R. Fundamentals of positron emission tomography and applications in preclinical drug development. J. Clin. Pharmacol. 41, 482–491 (2001).
Cunningham, V. J. et al. A method of studying pharmacokinetics in man at picolmolar drug concentrations. Br. J. Clin. Pharmacol. 32, 167–172 (1991).
Saleem, A. et al. Pharmacokinetic evaluation of N-(2-(dimethylamino)ethyl)acrideine-4-carboxamide in patients by positron emission tomography. J. Clin. Oncol. 19, 1421–1429 (2001).
Lappin, G. & Garner, R. C. In Handbook of Analytical Separations (ed. Smith, R. M.) (Elsevier, Amsterdam, in the press).
Garner, R. C. et al. Evaluation of Accelerator Mass Spectrometry in a human mass balance and pharmacokinetic study — experience with 14C-labelled -6-[amino(4-chlorophenyl)(1-methyl-1H-imidazole-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone (R115777), a farnesyl transferase inhibitor. Drug Metab. Dispos. 30, 823–829 (2002).
Gupta, N., Price, P. M. & Aboagye, E. O. PET for in vivo pharmacokinetic and pharmacodynamic measurements. Eur. J. Cancer. 38, 2094–2107 (2002).
Acknowledgements
We would like to thank Drs Ken Turteltaub and John Vogel at the Center for Acelerator Mass Spectrometry, Lawrence Livermore National Laboratories, USA, for introducing us to the AMS technique. Professors Bengt Langström and Mats Bergström at the PET Cenctre, Uppsala University, Sweden, for their assistance and for supplying Figure 4, and Dr Terry Jones, Manchester, UK, for his assistance on PET. In addition, the financial and scientific contributions of GlaxoSmithKline, Novartis, Pfizer, Janssen Pharmaceuticals and the University of York have proven invaluable to Xceleron Ltd.
Author information
Authors and Affiliations
Related links
Related links
DATABASE
Online Mendelian Inheritance in Man
FURTHER INFORMATION
Encyclopedia of Life Sciences
Rights and permissions
About this article
Cite this article
Lappin, G., Garner, R. Big physics, small doses: the use of AMS and PET in human microdosing of development drugs. Nat Rev Drug Discov 2, 233–240 (2003). https://doi.org/10.1038/nrd1037
Issue Date:
DOI: https://doi.org/10.1038/nrd1037
This article is cited by
-
Alternatives to animal models to study bacterial infections
Folia Microbiologica (2023)
-
Evaluation and Application of a PET Tracer in Preclinical and Phase 1 Studies to Determine the Brain Biodistribution of Minzasolmin (UCB0599)
Molecular Imaging and Biology (2023)
-
In vivo tracking of 14C thymidine labeled mesenchymal stem cells using ultra-sensitive accelerator mass spectrometry
Scientific Reports (2021)
-
Phase 0/microdosing approaches: time for mainstream application in drug development?
Nature Reviews Drug Discovery (2020)
-
Performing radiosynthesis in microvolumes to maximize molar activity of tracers for positron emission tomography
Communications Chemistry (2018)