Abstract
The connexin family of channel-forming proteins is present in every tissue type in the human anatomy. Connexins are best known for forming clustered intercellular channels, structurally known as gap junctions, where they serve to exchange members of the metabolome between adjacent cells. In their single-membrane hemichannel form, connexins can act as conduits for the passage of small molecules in autocrine and paracrine signalling. Here, we review the roles of connexins in health and disease, focusing on the potential of connexins as therapeutic targets in acquired and inherited diseases as well as wound repair, while highlighting the associated clinical challenges.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Revel, J. P. & Karnovsky, M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J. Cell Biol. 33, C7–C12 (1967).
Sohl, G. & Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 62, 228–232 (2004). This review describes the connexin gene family, including their structure.
Aasen, T., Mesnil, M., Naus, C. C., Lampe, P. D. & Laird, D. W. Gap junctions and cancer: communicating for 50 years. Nat. Rev. Cancer 16, 775–788 (2016). This is a comprehensive Review that summarizes the large body of work linking connexins, growth control and cancer.
Bruzzone, R., Hormuzdi, S. G., Barbe, M. T., Herb, A. & Monyer, H. Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl Acad. Sci. USA 100, 13644–13649 (2003). Here, the authors demonstrate that pannexins have channel-forming properties.
Esseltine, J. L. & Laird, D. W. Next-generation connexin and pannexin cell biology. Trends Cell Biol. 26, 944–955 (2016).
Srinivas, M., Verselis, V. K. & White, T. W. Human diseases associated with connexin mutations. Biochim. Biophys. Acta 1860, 192–201 (2018). An up-to-date review of diseases linked to inherited connexin gene mutations and mechanisms associated with their disruption of tissue homeostasis.
Laird, D. W., Naus, C. C. & Lampe, P. D. SnapShot: connexins and disease. Cell 170, 1260 (2017). This 'snapshot' gives a synopsis of how inherited connexin gene mutations are linked to disease.
Robertson, J. D. The occurrence of a subunit pattern in the unit membranes of club endings in mauthner cell synapses in goldfish brains. J. Cell Biol. 19, 201–221 (1963).
Benedetti, E. L. & Emmelot, P. Hexagonal array of subunits in tight junctions separated from isolated rat liver plasma membranes. J. Cell Biol. 38, 15–24 (1968).
Benedetti, E. L. & Emmelot, P. Electron microscopic observations on negatively stained plasma membranes isolated from rat liver. J. Cell Biol. 26, 299–305 (1965).
Sheridan, J. D. Electrophysiological evidence for low-resistance intercellular junctions in the early chick embryo. J. Cell Biol. 37, 650–659 (1968).
Kanno, Y. & Loewenstein, W. R. Cell-to-cell passage of large molecules. Nature 212, 629–630 (1966).
Loewenstein, W. R. Permeability of membrane junctions. Ann. NY Acad. Sci. 137, 441–472 (1966).
Paul, D. L. Molecular cloning of cDNA for rat liver gap junction protein. J. Cell Biol. 103, 123–134 (1986).
Kumar, N. M. & Gilula, N. B. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J. Cell Biol. 103, 767–776 (1986).
Beyer, E. C., Paul, D. L. & Goodenough, D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J. Cell Biol. 105, 2621–2629 (1987).
Kumar, N. M. & Gilula, N. B. Molecular biology and genetics of gap junction channels. Semin. Cell Biol. 3, 3–16 (1992).
Sohl, G. & Willecke, K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun. Adhes. 10, 173–180 (2003).
Willecke, K., Hennemann, H., Dahl, E., Jungbluth, S. & Heynkes, R. The diversity of connexin genes encoding gap junctional proteins. Eur. J. Cell Biol. 56, 1–7 (1991).
Angelillo-Scherrer, A. et al. Connexin 37 limits thrombus propensity by downregulating platelet reactivity. Circulation 124, 930–939 (2011).
Vaiyapuri, S. et al. Gap junctions and connexin hemichannels underpin hemostasis and thrombosis. Circulation 125, 2479–2491 (2012).
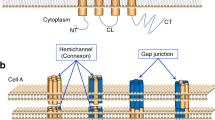
Goodenough, D. A., Goliger, J. A. & Paul, D. L. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 65, 475–502 (1996).
Laird, D. W. Life cycle of connexins in health and disease. Biochem. J. 394, 527–543 (2006).
Beyer, E. C. et al. Heteromeric mixing of connexins: compatibility of partners and functional consequences. Cell Commun. Adhes. 8, 199–204 (2001).
Koval, M. Pathways and control of connexin oligomerization. Trends Cell Biol. 16, 159–166 (2006).
Cottrell, G. T. & Burt, J. M. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim. Biophys. Acta 1711, 126–141 (2005).
Verselis, V., White, R. L., Spray, D. C. & Bennett, M. V. Gap junctional conductance and permeability are linearly related. Science 234, 461–464 (1986).
Goldberg, G. S., Lampe, P. D. & Nicholson, B. J. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat. Cell Biol. 1, 457–459 (1999).
White, T. W. & Bruzzone, R. Multiple connexin proteins in single intercellular channels: connexin compatibility and functional consequences. J. Bioener. Biomembr. 28, 339–350 (1996).
Maeda, S. et al. Structure of the connexin 26 gap junction channel at 3.5 Å resolution. Nature 458, 597–602 (2009). This high-resolution Cx26 structure study provided much more clarity to the arrangement of the transmembrane domains lining the connexon pore.
Bennett, B. C. et al. An electrostatic mechanism for Ca(2+)-mediated regulation of gap junction channels. Nat. Commun. 7, 8770 (2016).
Fallon, R. F. & Goodenough, D. A. Five-hour half-life of mouse liver gap-junction protein. J. Cell Biol. 90, 521–526 (1981).
Laird, D. W., Puranam, K. L. & Revel, J. P. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem. J. 273, 67–72 (1991).
Beardslee, M. A., Laing, J. G., Beyer, E. C. & Saffitz, J. E. Rapid turnover of connexin43 in the adult rat heart. Circ. Res. 83, 629–635 (1998).
Jiang, J. X., Paul, D. L. & Goodenough, D. A. Posttranslational phosphorylation of lens fiber connexin46: a slow occurrence. Invest. Ophthalmol. Vis. Sci. 34, 3558–3565 (1993).
Kelly, J. J., Shao, Q., Jagger, D. J. & Laird, D. W. Cx30 exhibits unique characteristics including a long half-life when assembled into gap junctions. J. Cell Sci. 128, 3947–3960 (2015).
Leybaert, L. et al. Connexins in cardiovascular and neurovascular health and disease: pharmacological implications. Pharmacol. Rev. 69, 396–478 (2017). This comprehensive review from many leaders in the gap junction field discusses the role of connexins in disease and how they are potential drug targets.
Delmar, M. et al. Connexins and disease. Cold Spring Harb. Perspect Biol. 10, a029348 (2017).
Laird, D. W. Closing the gap on autosomal dominant connexin-26 and connexin-43 mutants linked to human disease. J. Biol. Chem. 283, 2997–3001 (2008).
Koval, M., Molina, S. A. & Burt, J. M. Mix and match: investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS Lett. 588, 1193–1204 (2014).
Musil, L. S. & Goodenough, D. A. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell 74, 1065–1077 (1993).
Shaw, R. M. et al. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 128, 547–560 (2007).
Johnson, R. G. et al. Gap junctions assemble in the presence of cytoskeletal inhibitors, but enhanced assembly requires microtubules. Exp. Cell Res. 275, 67–80 (2002).
Giepmans, B. N. et al. Gap junction protein connexin-43 interacts directly with microtubules. Curr. Biol. 11, 1364–1368 (2001).
Li, H. et al. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J. Cell Biol. 134, 1019–1030 (1996).
Lopez, W. et al. Mechanism of gating by calcium in connexin hemichannels. Proc. Natl Acad. Sci. USA 113, E7986–E7995 (2016).
Rhett, J. M. & Gourdie, R. G. The perinexus: a new feature of Cx43 gap junction organization. Heart Rhythm 9, 619–623 (2011).
Vermij, S. H., Abriel, H. & van Veen, T. A. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc. Res. 113, 259–275 (2017).
Willebrords, J. et al. Connexins and their channels in inflammation. Crit. Rev. Biochem. Mol. Biol. 51, 413–439 (2016). This review focuses on the relationships between connexins and inflammation.
Gaietta, G. et al. Multicolor and electron microscopic imaging of connexin trafficking. Science 296, 503–507 (2002).
Lauf, U. et al. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc. Natl Acad. Sci. USA 99, 10446–10451 (2002).
Amsterdam, A., Josephs, R., Lieberman, M. E. & Lindner, H. R. Organization of intramembrane particles in freeze-cleaved gap junctions of rat graafian rollicles: optical-diffraction analysis. J. Cell Sci. 21, 93–105 (1976).
Jordan, K., Chodock, R., Hand, A. R. & Laird, D. W. The origin of annular junctions: a mechanism of gap junction internalization. J. Cell Sci. 114, 763–773 (2001).
Falk, M. M. et al. Degradation of endocytosed gap junctions by autophagosomal and endo-/lysosomal pathways: a perspective. J. Membr. Biol. 245, 465–476 (2012).
Norris, R. P., Baena, V. & Terasaki, M. Localization of phosphorylated connexin 43 using serial section immunogold electron microscopy. J. Cell Sci. 130, 1333–1340 (2017).
Qin, H., Shao, Q., Igdoura, S. A., Alaoui-Jamali, M. A. & Laird, D. W. Lysosomal and proteasomal degradation play distinct roles in the life cycle of Cx43 in gap junctional intercellular communication-deficient and -competent breast tumor cells. J. Biol. Chem. 278, 30005–30014 (2003).
Leithe, E., Sirnes, S., Fykerud, T., Kjenseth, A. & Rivedal, E. Endocytosis and post-endocytic sorting of connexins. Biochim. Biophys. Acta 1818, 1870–1879 (2011).
McLachlin, J. R., Caveney, S. & Kidder, G. M. Control of gap junction formation in early mouse embryos. Dev. Biol. 98, 155–164 (1983).
Davies, T. C., Barr, K. J., Jones, D. H., Zhu, D. & Kidder, G. M. Multiple members of the connexin gene family participate in preimplantation development of the mouse. Dev. Genet. 18, 234–243 (1996).
Boulay, A. C. et al. Hearing is normal without connexin30. J. Neurosci. 33, 430–434 (2013).
Kruger, O. et al. Defective vascular development in connexin 45-deficient mice. Development 127, 4179–4193 (2000).
Delorme, B. et al. Developmental regulation of connexin 40 gene expression in mouse heart correlates with the differentiation of the conduction system. Dev. Dyn. 204, 358–371 (1995).
Delmar, M. & Makita, N. Cardiac connexins, mutations and arrhythmias. Curr. Opin. Cardiol. 27, 236–241 (2012).
Merrifield, P. A. & Laird, D. W. Connexins in skeletal muscle development and disease. Semin. Cell Dev. Biol. 50, 67–73 (2016).
Rozental, R., Giaume, C. & Spray, D. C. Gap junctions in the nervous system. Brain Res. Rev. 32, 11–15 (2000).
Di, W. L., Rugg, E. L., Leigh, I. M. & Kelsell, D. P. Multiple epidermal connexins are expressed in different keratinocyte subpopulations including connexin 31. J. Invest. Dermatol. 117, 958–964 (2001).
Hennemann, H., Kozjek, G., Dahl, E., Nicholson, B. & Willecke, K. Molecular cloning of mouse connexins26 and -32: similar genomic organization but distinct promoter sequences of two gap junction genes. Eur. J. Cell Biol. 58, 81–89 (1992).
Fladmark, K. E. et al. Gap junctions and growth control in liver regeneration and in isolated rat hepatocytes. Hepatology 25, 847–855 (1997).
Stains, J. P. & Civitelli, R. Gap junctions in skeletal development and function. Biochim. Biophys. Acta 1719, 69–81 (2005).
Chow, L. & Lye, S. J. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am. J. Obstet. Gynecol. 170, 788–795 (1994).
Lefebvre, D. L., Piersanti, M., Bai, X. H., Chen, Z. Q. & Lye, S. J. Myometrial transcriptional regulation of the gap junction gene, connexin-43. Reprod. Fertil. Dev. 7, 603–611 (1995).
Kilarski, W. M., Rezapour, M., Backstrom, T., Roomans, G. M. & Ulmsten, U. Morphometric analysis of gap junction density in human myometrium at term. Acta Obstet. Gynecol. Scand. 73, 377–384 (1994).
Naus, C. C. & Laird, D. W. Implications and challenges of connexin connections to cancer. Nat. Rev. Cancer 10, 435–441 (2010).
Becker, D. L., Phillips, A. R., Duft, B. J., Kim, Y. & Green, C. R. Translating connexin biology into therapeutics. Semin. Cell Dev. Biol. 50, 49–58 (2016). This review, from the research teams that modulated Cx43 levels using antisense technologies and are currently exploiting hemichannel directed drugs, describes how these approaches can be utilized for therapeutic purposes.
Ghatnekar, G. S. et al. Connexin43 carboxyl-terminal peptides reduce scar progenitor and promote regenerative healing following skin wounding. Regen. Med. 4, 205–223 (2009).
Gourdie, R. G. et al. The unstoppable connexin43 carboxyl-terminus: new roles in gap junction organization and wound healing. Ann. NY Acad. Sci. 1080, 49–62 (2006).
Scheckenbach, K. E., Crespin, S., Kwak, B. R. & Chanson, M. Connexin channel-dependent signaling pathways in inflammation. J. Vasc. Res. 48, 91–103 (2011).
Loewenstein, W. R. & Kanno, Y. Intercellular communication and the control of tissue growth: lack of communication between cancer cells. Nature 209, 1248–1249 (1966).
Loewenstein, W. R. & Kanno, Y. Intercellular communication and tissue growth. I. Cancerous growth. J. Cell Biol. 33, 225–234 (1967).
Yotti, L. P., Chang, C. C. & Trosko, J. E. Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science 206, 1089–1091 (1979).
Zhu, D., Caveney, S., Kidder, G. M. & Naus, C. C. Transfection of C6 glioma cells with connexin 43 cDNA: analysis of expression, intercellular coupling, and cell proliferation. Proc. Natl Acad. Sci. USA 88, 1883–1887 (1991).
Naus, C. C., Zhu, D., Todd, S. D. & Kidder, G. M. Characteristics of C6 glioma cells overexpressing a gap junction protein. Cell. Mol. Neruobiol. 12, 163–175 (1992).
Zhu, D., Kidder, G. M., Caveney, S. & Naus, C. C. Growth retardation in glioma cells cocultured with cells overexpressing a gap junction protein. Proc. Natl Acad. Sci. USA 89, 10218–10221 (1992).
McLachlan, E., Shao, Q., Wang, H. L., Langlois, S. & Laird, D. W. Connexins act as tumor suppressors in three-dimensional mammary cell organoids by regulating differentiation and angiogenesis. Cancer Res. 66, 9886–9894 (2006).
Temme, A. et al. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr. Biol. 7, 713–716 (1997).
Evert, M., Ott, T., Temme, A., Willecke, K. & Dombrowski, F. Morphology and morphometric investigation of hepatocellular preneoplastic lesions and neoplasms in connexin32-deficient mice. Carcinogenesis 23, 697–703 (2002).
Fukumasu, H. et al. Higher susceptibility of spontaneous and NNK-induced lung neoplasms in connexin 43 deficient CD1 × AJ F1 mice: paradoxical expression of connexin 43 during lung carcinogenesis. Mol. Carcinog. 52, 497–506 (2013).
Stewart, M. K., Bechberger, J. F., Welch, I., Naus, C. C. & Laird, D. W. Cx26 knockout predisposes the mammary gland to primary mammary tumors in a DMBA-induced mouse model of breast cancer. Oncotarget 6, 37185–37199 (2015).
Mesnil, M. et al. Negative growth control of HeLa cells by connexin genes: connexin species specificity. Cancer Res. 55, 629–639 (1995).
Krutovskikh, V. A. et al. Differential effect of subcellular localization of communication impairing gap junction protein connexin43 on tumor cell growth in vivo. Oncogene 19, 505–513 (2000).
Yang, J. et al. Reciprocal positive regulation between Cx26 and PI3K/Akt pathway confers acquired gefitinib resistance in NSCLC cells via GJIC-independent induction of EMT. Cell Death Dis. 6, e1829 (2015).
Laird, D. W. The gap junction proteome and its relationship to disease. Trends Cell Biol. 20, 92–101 (2010).
Ruiz-Meana, M. et al. Mitochondrial connexin43 as a new player in the pathophysiology of myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 77, 325–333 (2008).
Sun, Y. et al. Connexin 43 interacts with Bax to regulate apoptosis of pancreatic cancer through a gap junction-independent pathway. Int. J. Oncol. 41, 941–948 (2012).
Boengler, K. & Schulz, R. Connexin 43 and mitochondria in cardiovascular health and disease. Adv. Exp. Med. Biol. 982, 227–246 (2017).
Dang, X., Doble, B. W. & Kardami, E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol. Cell Biochem. 242, 35–38 (2003).
Saez, J. C. & Leybaert, L. Hunting for connexin hemichannels. FEBS Lett. 588, 1205–1211 (2014).
Ito, A. et al. A role for heterologous gap junctions between melanoma and endothelial cells in metastasis. J. Clin. Invest. 105, 1189–1197 (2000).
Plante, I., Stewart, M. K., Barr, K., Allan, A. L. & Laird, D. W. Cx43 suppresses mammary tumor metastasis to the lung in a Cx43 mutant mouse model of human disease. Oncogene 30, 1681–1692 (2011).
Chen, Q. et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493–498 (2016).
Alonso, F. et al. Targeting endothelial connexin40 inhibits tumor growth by reducing angiogenesis and improving vessel perfusion. Oncotarget 7, 14015–14028 (2016).
Chan, D. K. & Chang, K. W. GJB2-associated hearing loss: systematic review of worldwide prevalence, genotype, and auditory phenotype. Laryngoscope 124, E34–E53 (2014).
Petit, C., Levilliers, J. & Hardelin, J. P. Molecular genetics of hearing loss. Annu. Rev. Genet. 35, 589–646 (2001).
Jamieson, S., Going, J. J., D'Arcy, R. & George, W. D. Expression of gap junction proteins connexin 26 and connexin 43 in normal human breast and in breast tumours. J. Pathol. 184, 37–43 (1998).
Marquez-Rosado, L., Singh, D., Rincon-Arano, H., Solan, J. L. & Lampe, P. D. CASK (LIN2) interacts with Cx43 in wounded skin and their coexpression affects cell migration. J. Cell Sci. 125, 695–702 (2012).
Dunn, C. A. & Lampe, P. D. Injury-triggered Akt phosphorylation of Cx43: a ZO-1-driven molecular switch that regulates gap junction size. J. Cell Sci. 127, 455–464 (2014).
Richards, T. S. et al. Protein kinase C spatially and temporally regulates gap junctional communication during human wound repair via phosphorylation of connexin43 on serine368. J. Cell Biol. 167, 555–562 (2004). This is a study of the Cx43 protein and phosphorylation level changes related to the temporal and spatial need for gap junction communication during wound healing.
Goliger, J. A. & Paul, D. L. Wounding alters epidermal connexin expression and gap junction-mediated intercellular communication. Mol. Biol. Cell 6, 1491–1501 (1995). This study shows how connexin (Cx26, Cx31.1 and Cx43) expression dramatically changes in wounded skin.
Lampe, P. D. et al. Cellular interaction of integrin alpha3beta1 with laminin 5 promotes gap junctional communication. J. Cell Biol. 143, 1735–1747 (1998).
Saitoh, M., Oyamada, M., Oyamada, Y., Kaku, T. & Mori, M. Changes in the expression of gap junction proteins (connexins) in hamster tongue epithelium during wound healing and carcinogenesis. Carcinogenesis 18, 1319–1328 (1997).
Coutinho, P., Qiu, C., Frank, S., Tamber, K. & Becker, D. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol. Int. 27, 525–541 (2003).
King, T. J. et al. Deficiency in the gap junction protein connexin32 alters p27Kip1 tumor suppression and MAPK activation in a tissue-specific manner. Oncogene 24, 1718–1726 (2005).
Kretz, M. et al. Altered connexin expression and wound healing in the epidermis of connexin-deficient mice. J. Cell Sci. 116, 3443–3452 (2003).
Qiu, C. et al. Targeting connexin43 expression accelerates the rate of wound repair. Curr. Biol. 13, 1697–1703 (2003).
Wang, C. M., Lincoln, J., Cook, J. E. & Becker, D. L. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes 56, 2809–2817 (2007).
Davidson, J. O., Green, C. R., Nicholson, L. F., Bennet, L. & Gunn, A. J. Deleterious effects of high dose connexin 43 mimetic peptide infusion after cerebral ischaemia in near-term fetal sheep. Int. J. Mol. Sci. 13, 6303–6319 (2012).
Nakano, Y. et al. Connexin43 knockdown accelerates wound healing but inhibits mesenchymal transition after corneal endothelial injury in vivo. Invest. Ophthalmol. Vis. Sci. 49, 93–104 (2008).
Grupcheva, C. N. et al. Improved corneal wound healing through modulation of gap junction communication using connexin43-specific antisense oligodeoxynucleotides. Invest. Opthamol. Vis. Sci. 53, 1130–1138 (2012). This is a key study showing that Cx43 antisense treatment to a scrape corneal wound rat model causes a significant reduction in wound area.
Moore, K. et al. A synthetic connexin 43 mimetic peptide augments corneal wound healing. Exp. Eye Res. 115, 178–188 (2013). This study effectively shows that microencapsulated aCT1 significantly improves rat corneal surgical wound closure.
Moore, K., Ghatnekar, G., Gourdie, R. G. & Potts, J. D. Impact of the controlled release of a connexin 43 peptide on corneal wound closure in an STZ model of type I diabetes. PLOS ONE 9, e86570 (2014).
Spray, D. C. & Burt, J. M. Structure-activity relations of the cardiac gap junction channel. Am. J. Physiol. 258, C195–C205 (1990).
Jalife, J., Morley, G. E. & Vaidya, D. Connexins and impulse propagation in the mouse heart. J. Cardiovasc. Electrophysiol. 10, 1649–1663 (1999).
Kirchhoff, S. et al. Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Curr. Biol. 8, 299–302 (1998).
Gutstein, D. E. et al. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ. Res. 88, 333–339 (2001).
Simon, A. M., Goodenough, D. A. & Paul, D. L. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr. Biol. 8, 295–298 (1998).
Severs, N. J., Bruce, A. F., Dupont, E. & Rothery, S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc. Res. 80, 9–19 (2008).
Guerrero, P. A. et al. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J. Clin. Invest. 99, 1991–1998 (1997).
Morley, G. E. et al. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J. Cardiovasc. Electrophysiol. 10, 1361–1375 (1999).
Hesketh, G. G. et al. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ. Res. 106, 1153–1163 (2010).
Hichri, E., Abriel, H. & Kucera, J. P. Distribution of cardiac sodium channels in clusters potentiates ephaptic interactions in the intercalated disc. J. Physiol. 596, 563–589 (2018).
Veeraraghavan, R. et al. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study. Pflugers Arch. 467, 2093–2105 (2015).
Raisch, T. B. et al. Intercalated disk extracellular nanodomain expansion in patients with atrial fibrillation. Front. Physiol. 9, 398 (2018).
Beardslee, M. A. et al. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ. Res. 87, 656–662 (2000).
Murry, C. E., Jennings, R. B. & Reimer, K. A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74, 1124–1136 (1986).
Jain, S. K., Schuessler, R. B. & Saffitz, J. E. Mechanisms of delayed electrical uncoupling induced by ischemic preconditioning. Circ. Res. 92, 1138–1144 (2003).
Schulz, R. & Heusch, G. Connexin 43 and ischemic preconditioning. Cardiovasc. Res. 62, 335–344 (2004).
Martins-Marques, T., Anjo, S. I., Pereira, P., Manadas, B. & Girao, H. Interacting network of the gap junction (GJ) protein connexin43 (Cx43) is modulated by ischemia and reperfusion in the heart. Mol. Cell. Proteomics 14, 3040–3055 (2015).
Flenniken, A. M. et al. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development 132, 4375–4386 (2005).
Zhao, H. B. Hypothesis of K(+)-recycling defect is not a primary deafness mechanism for Cx26 (GJB2) deficiency. Front. Mol. Neurosci. 10, 162 (2017).
Verselis, V. K. Connexin hemichannels and cochlear function. Neurosci. Lett. https://doi.org/10.1016/j.neulet.2017.09.020 (2017).
Mittal, R. et al. Signaling in the auditory system: implications in hair cell regeneration and hearing function. J. Cell. Physiol. 232, 2710–2721 (2017).
Rubinos, C., Villone, K., Mhaske, P. V., White, T. W. & Srinivas, M. Functional effects of Cx50 mutations associated with congenital cataracts. Am. J. Physiol. Cell Physiol. 306, C212–C220 (2014).
Pal, J. D. et al. Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am. J. Physiol. Cell Physiol. 279, C596–C602 (2000).
Kannabiran, C. & Balasubramanian, D. Molecular genetics of cataract. Indian J. Ophthalmol. 48, 5–13 (2000).
Bergoffen, J. et al. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262, 2039–2042 (1993).
Ionasescu, V., Searby, C. & Ionasescu, R. Point mutations of the connexin32 (GJB1) gene in X-linked dominant Charcot-Marie-Tooth neuropathy. Hum. Mol. Genet. 3, 355–358 (1994).
Orthmann-Murphy, J. L. et al. Hereditary spastic paraplegia is a novel phenotype for GJA12/GJC2 mutations. Brain 132, 426–438 (2009).
Kim, M. S., Gloor, G. B. & Bai, D. The distribution and functional properties of Pelizaeus-Merzbacher-like disease-linked Cx47 mutations on Cx47/Cx47 homotypic and Cx47/Cx43 heterotypic gap junctions. Biochem. J. 452, 249–258 (2013).
Ressot, C. & Bruzzone, R. Connexin channels in Schwann cells and the development of the X-linked form of Charcot-Marie-Tooth disease. Brain Res. Rev. 32, 192–202 (2000).
Lilly, E., Sellitto, C., Milstone, L. M. & White, T. W. Connexin channels in congenital skin disorders. Semin. Cell Dev. Biol. 50, 4–12 (2016).
Lee, J. R. & White, T. W. Connexin-26 mutations in deafness and skin disease. Expert Rev. Mol. Med. 11, e35 (2009).
Churko, J. M. & Laird, D. W. Gap junction remodeling in skin repair following wounding and disease. Physiology 28, 190–198 (2013).
Mese, G. et al. The Cx26-G45E mutation displays increased hemichannel activity in a mouse model of the lethal form of keratitis-ichthyosis-deafness syndrome. Mol. Biol. Cell 22, 4776–4786 (2011).
Paznekas, W. A. et al. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum. Mutat. 30, 724–733 (2009).
Paznekas, W. A. et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 72, 408–418 (2003). This report describes how several mutations in the gene encoding Cx43 are causal of oculodentodigital dysplasia.
Reaume, A. G. et al. Cardiac malformation in neonatal mice lacking connexin43. Science 267, 1831–1834 (1995).
Roberts, J. D. et al. Targeted deep sequencing reveals no evidence for somatic mosaicism in atrial fibrillation. Circ. Cardiovasc. Genet. 8, 50–57 (2015).
Gollob, M. H. et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N. Engl. J. Med. 354, 2677–2688 (2006).
Kelly, J. J., Simek, J. & Laird, D. W. Mechanisms linking connexin mutations to human diseases. Cell Tissue Res. 360, 701–721 (2015).
Hagendorff, A., Schumacher, B., Kirchhoff, S., Luderitz, B. & Willecke, K. Conduction disturbances and increased atrial vulnerability in connexin40-deficient mice analyzed by transesophageal stimulation. Circulation 99, 1508–1515 (1999).
Chan, D. K., Schrijver, I. & Chang, K. W. Connexin-26-associated deafness: phenotypic variability and progression of hearing loss. Genet. Med. 12, 174–181 (2010).
Martinez, A. D., Acuna, R., Figueroa, V., Maripillan, J. & Nicholson, B. Gap-junction channels dysfunction in deafness and hearing loss. Antioxid. Redox Signal. 11, 309–322 (2009).
Tekin, M., Arnos, K. S. & Pandya, A. Advances in hereditary deafness. Lancet 358, 1082–1090 (2001).
Haapaniemi, E., Botla, S., Persson, J., Schmierer, B. & Taipale, J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat. Med. 24, 927–930 (2018).
Riquelme, M. A., Kar, R., Gu, S. & Jiang, J. X. Antibodies targeting extracellular domain of connexins for studies of hemichannels. Neuropharmacology 75, 525–532 (2013).
Zhou, J. Z. et al. Osteocytic connexin hemichannels suppress breast cancer growth and bone metastasis. Oncogene 35, 5597–5607 (2016).
Coutinho, P. et al. Limiting burn extension by transient inhibition of connexin43 expression at the site of injury. Br. J. Plast. Surg. 58, 658–667 (2005).
Cronin, M., Anderson, P. N., Cook, J. E., Green, C. R. & Becker, D. L. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol. Cell Neurosci. 39, 152–160 (2008). This report reveals that Cx43 antisense application in two models of rat spinal cord injury reduces swelling, tissue disruption, astrocytic GFAP upregulation and neutrophil extravasation.
O'Quinn, M. P., Palatinus, J. A., Harris, B. S., Hewett, K. W. & Gourdie, R. G. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ. Res. 108, 704–715 (2011). Here, aCT1 is shown to significantly reduce lateralization of Cx43 in a mouse model of heart cryoinjury.
Palatinus, J. A., Rhett, J. M. & Gourdie, R. G. The connexin43 carboxyl terminus and cardiac gap junction organization. Biochim. Biophys. Acta 1818, 1831–1843 (2012).
Giepmans, B. N. & Moolenaar, W. H. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr. Biol. 8, 931–934 (1998).
Toyofuku, T. et al. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J. Biol. Chem. 273, 12725–12731 (1998).
Grek, C. L. et al. Topical administration of a connexin43-based peptide augments healing of chronic neuropathic diabetic foot ulcers: a multicenter, randomized trial. Wound Repair Regen. 23, 203–212 (2015).
Ghatnekar, G. S., Grek, C. L., Armstrong, D. G., Desai, S. C. & Gourdie, R. G. The effect of a connexin43-based peptide on the healing of chronic venous leg ulcers: a multicenter, randomized trial. J. Invest. Dermatol. 135, 289–298 (2015). This phase II clinical trial shows that aCT1 treatment causes a significantly greater reduction in the per cent ulcer area and a doubling of the incidence of complete wound closure.
Montgomery, J., Ghatnekar, G. S., Grek, C. L., Moyer, K. E. & Gourdie, R. G. Connexin 43-based therapeutics for dermal wound healing. Int. J. Mol. Sci. 19, E1778 (2018).
Grek, C. L. et al. A multicenter randomized controlled trial evaluating a Cx43-mimetic peptide in cutaneous scarring. J. Invest. Dermatol. 137, 620–630 (2017). This report describes the results from a phase II clinical trial on the effect of aCT1 after laparoscopic surgery and shows improvements in scar pigmentation, thickness, surface roughness and mechanical suppleness.
Hunter, A. W., Barker, R. J., Zhu, C. & Gourdie, R. G. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol. Biol. Cell 16, 5686–5698 (2005).
Rhett, J. M., Jourdan, J. & Gourdie, R. G. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol. Biol. Cell 22, 1516–1528 (2011).
Mendoza-Naranjo, A. et al. Overexpression of the gap junction protein Cx43 as found in diabetic foot ulcers can retard fibroblast migration. Cell Biol. Int. 36, 661–667 (2012).
Palatinus, J. A. & Gourdie, R. G. Diabetes increases cryoinjury size with associated effects on Cx43 gap junction function and phosphorylation in the mouse heart. J. Diabetes Res. 2016, 8789617 (2016).
Wong, P. et al. The role of connexins in wound healing and repair: novel therapeutic approaches. Front. Physiol. 7, 596 (2016).
Deva, N. C., Zhang, J., Green, C. R. & Danesh-Meyer, H. V. Connexin43 modulation inhibits scarring in a rabbit eye glaucoma trabeculectomy model. Inflammation 35, 1276–1286 (2012).
Ormonde, S. et al. Regulation of connexin43 gap junction protein triggers vascular recovery and healing in human ocular persistent epithelial defect wounds. J. Membr. Biol. 245, 381–388 (2012).
Goadsby, P. J. Emerging therapies for migraine. Nat. Clin. Pract. Neurol. 3, 610–619 (2007).
Damodaram, S., Thalakoti, S., Freeman, S. E., Garrett, F. G. & Durham, P. L. Tonabersat inhibits trigeminal ganglion neuronal-satellite glial cell signaling. Headache 49, 5–20 (2009).
Dahlof, C. G., Hauge, A. W. & Olesen, J. Efficacy and safety of tonabersat, a gap-junction modulator, in the acute treatment of migraine: a double-blind, parallel-group, randomized study. Cephalalgia 29, 7–16 (2009).
Silberstein, S. D. et al. Tonabersat, a gap-junction modulator: efficacy and safety in two randomized, placebo-controlled, dose-ranging studies of acute migraine. Cephalalgia 29, 17–27 (2009).
Silberstein, S. D. Tonabersat, a novel gap-junction modulator for the prevention of migraine. Cephalalgia 29, 28–35 (2009).
Kim, Y. et al. Tonabersat prevents inflammatory damage in the central nervous system by blocking connexin43 hemichannels. Neurotherapeutics 14, 1148–1165 (2017).
Mao, Y. et al. Characterisation of Peptide5 systemic administration for treating traumatic spinal cord injured rats. Exp. Brain Res. 235, 3033–3048 (2017).
Danesh-Meyer, H. V. et al. Connexin43 mimetic peptide reduces vascular leak and retinal ganglion cell death following retinal ischaemia. Brain 135, 506–520 (2012). This study reports that Peptide5 treatment increases neuronal rescue after retinal IRI.
Chen, Y. S. et al. Intravitreal injection of lipoamino acid-modified connexin43 mimetic peptide enhances neuroprotection after retinal ischemia. Drug Deliv. Transl Res. 5, 480–488 (2015).
Davidson, J. O. et al. Connexin hemichannel blockade improves outcomes in a model of fetal ischemia. Ann. Neurol. 71, 121–132 (2012).
Davidson, J. O. et al. Connexin hemichannel blockade is neuroprotective after asphyxia in preterm fetal sheep. PLOS ONE 9, e96558 (2014).
Galinsky, R. et al. Connexin hemichannel blockade improves survival of striatal GABA-ergic neurons after global cerebral ischaemia in term-equivalent fetal sheep. Sci. Rep. 7, 6304 (2017). Here, Peptide5 infused for 24 hours following global ischaemia is shown to improve survival of striatal GABAergic neurons in sheep.
Chen, Y. S., Green, C. R., Wang, K., Danesh-Meyer, H. V. & Rupenthal, I. D. Sustained intravitreal delivery of connexin43 mimetic peptide by poly(D,L-lactide-co-glycolide) acid micro- and nanoparticles—closing the gap in retinal ischaemia. Eur. J. Pharm. Biopharm. 95, 378–386 (2015).
Guo, C. X. et al. Connexin43 mimetic peptide improves retinal function and reduces inflammation in a light-damaged albino rat model. Invest. Ophthalmol. Vis. Sci. 57, 3961–3973 (2016). In this report, Peptide5 is found to significantly preserve photoreceptoral and postphotoreceptoral neurons in bright-light-treated albino rats.
Kim, Y. et al. Characterizing the mode of action of extracellular connexin43 channel blocking mimetic peptides in an in vitro ischemia injury model. Biochim. Biophys. Acta 1861, 68–78 (2017).
Danesh-Meyer, H. V., Zhang, J., Acosta, M. L., Rupenthal, I. D. & Green, C. R. Connexin43 in retinal injury and disease. Prog. Retin. Eye Res. 51, 41–68 (2016). Here, the authors review the evidence that Cx43 hemichannels may be acting as a pathological pore in retinal injury and disease.
Mugisho, O. O. et al. The inflammasome pathway is amplified and perpetuated in an autocrine manner through connexin43 hemichannel mediated ATP release. Biochim. Biophys. Acta 1862, 385–393 (2018).
Tonkin, R. S. et al. Attenuation of mechanical pain hypersensitivity by treatment with Peptide5, a connexin-43 mimetic peptide, involves inhibition of NLRP3 inflammasome in nerve-injured mice. Exp. Neurol. 300, 1–12 (2018). In this study, Peptide5 treatment results in significantly reduced Cx43 and microglial and astrocyte activity in the dorsal horn of the spinal cord in chronic constriction injury mice.
Cea, L. A. et al. De novo expression of connexin hemichannels in denervated fast skeletal muscles leads to atrophy. Proc. Natl Acad. Sci. USA 110, 16229–16234 (2013).
Cea, L. A. et al. Dexamethasone-induced muscular atrophy is mediated by functional expression of connexin-based hemichannels. Biochim. Biophys. Acta 1862, 1891–1899 (2016).
Yi, C. et al. Astroglial connexin43 contributes to neuronal suffering in a mouse model of Alzheimer's disease. Cell Death Differ. 23, 1691–1701 (2016).
Obert, E. et al. Targeting the tight junction protein, zonula occludens-1, with the connexin43 mimetic peptide, alphaCT1, reduces VEGF-dependent RPE pathophysiology. J. Mol. Med. 95, 535–552 (2017). Here, aCT1 delivered via eye drops is shown to reduce light-induced retinal pigment epithelium damage.
Duchêne, A. et al. Impact of astroglial connexins on modafinil pharmacological properties. Sleep 39, 1283–1292 (2016). In this report, flecainide enhances the wake-promoting and procognitive effects of modafinil in narcoleptic orexin knockout mice.
Liu, X. et al. The psychostimulant modafinil enhances gap junctional communication in cortical astrocytes. Neuropharmacology (2013).
Cruikshank, S. J. et al. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc. Natl Acad. Sci. USA 101, 12364–12369 (2004).
Picoli, C. et al. Human connexin channel specificity of classical and new gap junction inhibitors. J. Biomol. Screen. 17, 1339–1347 (2012).
Jeanson, T. et al. Potentiation of amitriptyline anti-hyperalgesic-like action by astroglial connexin 43 inhibition in neuropathic rats. Sci. Rep. 6, 38766 (2016).
Dhein, S. et al. A new synthetic antiarrhythmic peptide reduces dispersion of epicardial activation recovery interval and diminishes alterations of epicardial activation patterns induced by regional ischemia. A mapping study. Naunyn Schmiedebergs Arch. Pharmacol. 350, 174–184 (1994).
Lin, X., Zemlin, C., Hennan, J. K., Petersen, J. S. & Veenstra, R. D. Enhancement of ventricular gap-junction coupling by rotigaptide. Cardiovasc. Res. 79, 416–426 (2008). Here, rotigaptide is demonstrated to increase gap junction coupling in ventricular cardiomyocytes.
Jorgensen, N. R. et al. The antiarrhythmic peptide analog rotigaptide (ZP123) stimulates gap junction intercellular communication in human osteoblasts and prevents decrease in femoral trabecular bone strength in ovariectomized rats. Endocrinology 146, 4745–4754 (2005).
Clarke, T. C., Thomas, D., Petersen, J. S., Evans, W. H. & Martin, P. E. The antiarrhythmic peptide rotigaptide (ZP123) increases gap junction intercellular communication in cardiac myocytes and HeLa cells expressing connexin 43. Br. J. Pharmacol. 147, 486–495 (2006).
Hsieh, Y. C. et al. Gap junction modifier rotigaptide decreases the susceptibility to ventricular arrhythmia by enhancing conduction velocity and suppressing discordant alternans during therapeutic hypothermia in isolated rabbit hearts. Heart Rhythm. 13, 251–261 (2016). This study demonstrates that the gap junction modifier rotigaptide protects rabbit hearts from ventricular arrhythmias.
Ueda, N., Yamamoto, M., Honjo, H., Kodama, I. & Kamiya, K. The role of gap junctions in stretch-induced atrial fibrillation. Cardiovasc. Res. 104, 364–370 (2014).
Rossman, E. I. et al. The gap junction modifier, GAP-134 [(2S,4R)-1-(2-aminoacetyl)-4-benzamido-pyrrolidine-2-carboxylic acid], improves conduction and reduces atrial fibrillation/flutter in the canine sterile pericarditis model. J. Pharmacol. Exp. Ther. 329, 1127–1133 (2009).
Ng, F. S. et al. Enhancement of gap junction function during acute myocardial infarction modifies healing and reduces late ventricular arrhythmia susceptibility. JACC Clin. Electrophysiol. 2, 574–582 (2016).
Pedersen, C. M. et al. Rotigaptide protects the myocardium and arterial vasculature from ischaemia reperfusion injury. Br. J. Clin. Pharmacol. 81, 1037–1045 (2016).
Skyschally, A., Walter, B., Schultz Hansen, R. & Heusch, G. The antiarrhythmic dipeptide ZP1609 (danegaptide) when given at reperfusion reduces myocardial infarct size in pigs. Naunyn Schmiedebergs Arch. Pharmacol. 386, 383–391 (2013).
Boengler, K., Bulic, M., Schreckenberg, R., Schluter, K. D. & Schulz, R. The gap junction modifier ZP1609 decreases cardiomyocyte hypercontracture following ischaemia/reperfusion independent from mitochondrial connexin 43. Br. J. Pharmacol. 174, 2060–2073 (2017).
Laurent, G. et al. Effects of chronic gap junction conduction-enhancing antiarrhythmic peptide GAP-134 administration on experimental atrial fibrillation in dogs. Circ. Arrhythm. Electrophysiol. 2, 171–178 (2009).
Engstrom, T. et al. Danegaptide for primary percutaneous coronary intervention in acute myocardial infarction patients: a phase 2 randomised clinical trial. Heart https://doi.org/10.1136/heartjnl-2017-312774 (2018).
Huang, G. Y. et al. Alteration in connexin 43 gap junction gene dosage impairs conotruncal heart development. Dev. Biol. 198, 32–44 (1998).
Lo, C. W., Waldo, K. L. & Kirby, M. L. Gap junction communication and the modulation of cardiac neural crest cells. Trends Cardiovasc. Med. 9, 63–69 (1999).
Huang, G. Y. et al. Gap junction-mediated cell-cell communication modulates mouse neural crest migration. J. Cell Biol. 143, 1725–1734 (1998).
Li, W. E. et al. An essential role for connexin43 gap junctions in mouse coronary artery development. Development 129, 2031–2042 (2002).
Dobrowolski, R. & Willecke, K. Connexin-caused genetic diseases and corresponding mouse models. Antioxid. Redox Signal. 11, 283–295 (2009).
Gabriel, H. D. et al. Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J. Cell Biol. 140, 1453–1461 (1998).
Winterhager, E. et al. Connexin expression patterns in human trophoblast cells during placental development. Placenta 20, 627–638 (1999).
Nishii, K., Shibata, Y. & Kobayashi, Y. Connexin mutant embryonic stem cells and human diseases. World J. Stem Cells 6, 571–578 (2014).
Denoyelle, F. et al. Clinical features of the prevalent form of childhood deafness, DFNB1, due to a connexin-26 gene defect: implications for genetic counselling. Lancet 353, 1298–1303 (1999).
Cohn, E. S. & Kelley, P. M. Clinical phenotype and mutations in connexin 26 (DFNB1/GJB2), the most common cause of childhood hearing loss. Am. J. Med. Genet. 89, 130–136 (1999).
Teubner, B. et al. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum. Mol. Genet. 12, 13–21 (2003).
Smith, F. J., Morley, S. M. & McLean, W. H. A novel connexin 30 mutation in Clouston syndrome. J. Invest. Dermatol. 118, 530–532 (2002).
Bosen, F. et al. The Clouston syndrome mutation connexin30 A88V leads to hyperproliferation of sebaceous glands and hearing impairments in mice. FEBS Lett. 588, 1795–1801 (2014).
Huang, D., Chen, Y. S., Green, C. R. & Rupenthal, I. D. Hyaluronic acid coated albumin nanoparticles for targeted peptide delivery in the treatment of retinal ischaemia. Biomaterials 168, 10–23 (2018).
Xu, L. et al. Design and characterization of a human monoclonal antibody that modulates mutant connexin 26 hemichannels implicated in deafness and skin disorders. Front. Mol. Neurosci. 10, 298 (2017).
Panchin, Y. et al. A ubiquitous family of putative gap junction molecules. Curr. Biol. 10, R473–R474 (2000). This paper reports the discovery of a new family of putative channel-forming proteins called pannexins.
Baranova, A. et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83, 706–716 (2004).
Panchin, Y. V. Evolution of gap junction proteins—the pannexin alternative. J. Exp. Biol. 208, 1415–1419 (2005).
Bond, S. R. & Naus, C. C. The pannexins: past and present. Front. Physiol. 5, 58 (2014).
Lohman, A. W. & Isakson, B. E. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett. 588, 1379–1388 (2014).
Isakson, B. E. & Thompson, R. J. Pannexin-1 as a potentiator of ligand-gated receptor signaling. Channels 8, 118–123 (2014).
Penuela, S. et al. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 120, 3772–3783 (2007).
Boassa, D. et al. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J. Biol. Chem. 282, 31733–31743 (2007).
Penuela, S., Bhalla, R., Nag, K. & Laird, D. W. Glycosylation regulates pannexin intermixing and cellular localization. Mol. Biol. Cell. 20, 4313–4323 (2009).
Ambrosi, C. et al. Pannexin1 and pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J. Biol. Chem. 285, 24420–24431 (2010).
Wang, J. et al. The membrane protein pannexin1 forms two open-channel conformations depending on the mode of activation. Sci. Signal. 7, ra69 (2014).
Thompson, R. J. & Macvicar, B. A. Connexin and pannexin hemichannels of neurons and astrocytes. Channels 2, 81–86 (2008).
Vanden Abeele, F. et al. Functional implications of calcium permeability of the channel formed by pannexin 1. J. Cell Biol. 174, 535–546 (2006).
Bhalla-Gehi, R., Penuela, S., Churko, J. M., Shao, Q. & Laird, D. W. Pannexin1 and pannexin3 delivery, cell surface dynamics, and cytoskeletal interactions. J. Biol. Chem. 285, 9147–9160 (2010).
Boyce, A. K., Wicki-Stordeur, L. E. & Swayne, L. A. Powerful partnership: crosstalk between pannexin 1 and the cytoskeleton. Front. Physiol. 5, 27 (2014).
Bao, L., Locovei, S. & Dahl, G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 572, 65–68 (2004). This study reports the identification of ATP as a permeant of pannexin channels.
Chekeni, F. B. et al. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature 467, 863–867 (2010). Here, the authors demonstrate that ATP and uridine-5′-triphosphate release through caspase-cleaved pannexin 1 channels serves a functional role in apoptosis.
Whyte-Fagundes, P. & Zoidl, G. Mechanisms of pannexin1 channel gating and regulation. Biochim. Biophys. Acta 1860, 65–71 (2018).
Sandilos, J. K. et al. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C terminal autoinhibitory region. J. Biol. Chem. 287, 11303–11311 (2012).
Qu, Y. et al. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J. Immunol. 186, 6553–6561 (2011).
Chiu, Y. H. et al. A quantized mechanism for activation of pannexin channels. Nat. Commun. 8, 14324 (2017).
Gehi, R., Shao, Q. & Laird, D. W. Pathways regulating the trafficking and turnover of pannexin1 protein and the role of the C-terminal domain. J. Biol. Chem. 286, 27639–27653 (2011).
Boyce, A. K. J., Epp, A. L., Nagarajan, A. & Swayne, L. A. Transcriptional and post-translational regulation of pannexins. Biochim. Biophys. Acta 1860, 72–82 (2018).
Shao, Q. et al. A germline variant in the PANX1 gene has reduced channel function and is associated with multisystem dysfunction. J. Biol. Chem. 291, 12432–12443 (2016).
Penuela, S. et al. Loss of pannexin 1 attenuates melanoma progression by reversion to a melanocytic phenotype. J. Biol. Chem. 287, 29184–29193 (2012).
Kim, Y. et al. Connexins and pannexins in cerebral ischemia. Biochim. Biophys. Acta 1860, 224–236 (2018).
Dong, F., Yang, X. J., Jiang, T. B. & Chen, Y. Ischemia triggered ATP release through pannexin-1 channel by myocardial cells activates sympathetic fibers. Microvasc. Res. 104, 32–37 (2016).
Thompson, R. J. Pannexin channels and ischaemia. J. Physiol. 593, 3463–3470 (2014).
Freitas-Andrade, M., Bechberger, J. F., MacVicar, B. A., Viau, V. & Naus, C. C. Pannexin1 knockout and blockade reduces ischemic stroke injury in female, but not in male mice. Oncotarget 8, 36973–36983 (2017).
Good, M. E. et al. Endothelial cell pannexin1 modulates severity of ischemic stroke by regulating cerebral inflammation and myogenic tone. JCI Insight 3, 96272 (2018).
Santiago, M. F. et al. Targeting pannexin1 improves seizure outcome. PLOS ONE 6, e25178 (2011).
Gulbransen, B. D. et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med. 18, 600–604 (2012).
Chen, S. P. et al. Inhibition of the P2X7-PANX1 complex suppresses spreading depolarization and neuroinflammation. Brain 140, 1643–1656 (2017).
Seror, C. et al. Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J. Exp. Med. 208, 1823–1834 (2011).
Moon, P. M. et al. Deletion of Panx3 prevents the development of surgically induced osteoarthritis. J. Mol. Med. 93, 845–856 (2015).
Thompson, R. J. et al. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 322, 1555–1559 (2008).
Dossi, E. et al. Pannexin-1 channels contribute to seizure generation in human epileptic brain tissue and in a mouse model of epilepsy. Sci. Transl Med. 10, eaar3796 (2018).
Aquilino, M. S., Whyte-Fagundes, P., Zoidl, G. & Carlen, P. L. Pannexin-1 channels in epilepsy. Neurosci. Lett. https://doi.org/10.1016/j.neulet.2017.09.004 (2017).
Penuela, S., Harland, L., Simek, J. & Laird, D. W. Pannexin channels and their links to human disease. Biochem. J. 461, 371–381 (2014).
Burma, N. E. et al. Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat. Med. 23, 355–360 (2017).
Xu, J., Chen, L. & Li, L. Pannexin hemichannels: a novel promising therapy target for oxidative stress related diseases. J. Cell. Physiol. 233, 2075–2090 (2018).
Willebrords, J., Maes, M., Crespo Yanguas, S. & Vinken, M. Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacol. Ther. 180, 144–160 (2017).
Jiang, J. X. & Penuela, S. Connexin and pannexin channels in cancer. BMC Cell Biol. 17 (Suppl. 1), 12 (2016).
Kranz, K. et al. Expression of pannexin1 in the outer plexiform layer of the mouse retina and physiological impact of its knock-out. J. Comp. Neurol. 521, 1119–1135 (2012).
Bargiotas, P. et al. Pannexins in ischemia-induced neurodegeneration. Proc. Natl Acad. Sci. USA. 108, 20772–20777 (2011).
Silverman, W., Locovei, S. & Dahl, G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am. J. Physiol. Cell Physiol. 295, C761–C767 (2008).
Poon, I. K. et al. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 507, 329–334 (2014).
Good, M. E. et al. Pannexin 1 channels as an unexpected new target of the anti-hypertensive drug spironolactone. Circ. Res. 122, 606–615 (2018).
Billaud, M. et al. A molecular signature in the pannexin1 intracellular loop confers channel activation by the α1 adrenoreceptor in smooth muscle cells. Sci. Signal. 8, ra17 (2015).
Schulte, J., Sepp, K. J., Wu, C., Hong, P. & Littleton, J. T. High-content chemical and RNAi screens for suppressors of neurotoxicity in a Huntington's disease model. PLOS ONE 6, e23841 (2011).
Michalski, K. & Kawate, T. Carbenoxolone inhibits pannexin1 channels through interactions in the first extracellular loop. J. Gen. Physiol. 147, 165–174 (2016).
Laird, D. W. & Revel, J. P. Biochemical and immunochemical analysis of the arrangement of connexin43 in rat heart gap junction membranes. J. Cell Sci. 97, 109–117 (1990).
Gemel, J., Lin, X., Veenstra, R. D. & Beyer, E. C. N-Terminal residues in Cx43 and Cx40 determine physiological properties of gap junction channels, but do not influence heteromeric assembly with each other or with Cx26. J. Cell Sci. 119, 2258–2268 (2006).
John, S. A. & Revel, J. P. Connexon integrity is maintained by non-covalent bonds: intramolecular disulfide bonds link the extracellular domains in rat connexin-43. Biochem. Biophys. Res. Commun. 178, 1312–1318 (1991).
Morley, G. E., Ek-Vitorin, J. F., Taffet, S. M. & Delmar, M. Structure of connexin43 and its regulation by pHi. J. Cardiovasc. Electrophysiol. 8, 939–951 (1997).
Solan, J. L. & Lampe, P. D. Connexin43 phosphorylation: structural changes and biological effects. Biochem. J. 419, 261–272 (2009).
Leithe, E., Mesnil, M. & Aasen, T. The connexin 43 C-terminus: a tail of many tales. Biochim. Biophys. Acta 1860, 48–64 (2018).
Bai, D., Yue, B. & Aoyama, H. Crucial motifs and residues in the extracellular loops influence the formation and specificity of connexin docking. Biochim. Biophys. Acta 1860, 9–21 (2018).
Sonntag, S. et al. Mouse lens connexin23 (Gje1) does not form functional gap junction channels but causes enhanced ATP release from HeLa cells. Eur. J. Cell Biol. 88, 65–77 (2009).
Leo-Macias, A., Agullo-Pascual, E. & Delmar, M. The cardiac connexome: non-canonical functions of connexin43 and their role in cardiac arrhythmias. Semin. Cell Dev. Biol. 50, 13–21 (2016).
Acknowledgements
Given the >20,000 reports on connexins and pannexins and reference number limitations, the authors apologize for not including many primary articles and for summarizing many exciting findings by citing reviews. Work in the authors' laboratories is supported by a US National Institutes of Health grant (GM55632) to P.D.L. and the Canadian Institutes of Health Research (130530, 123228, 148630 and 148584) and Canada Research Chair Program to D.W.L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
P.D.L. is a non-paid member of the scientific advisory board of FirstString Research and has been granted stock options (currently no value) for his service. D.W.L. received a small 1-year grant from Zealand Pharma in 2016 to test potential pannexin 1-modulating peptides.
Glossary
- Connexins
-
Tetraspanning membrane proteins that are the molecular constituent of gap junctions.
- Hemichannel
-
A connexon that has the ability to open for the passage of small molecules.
- Ischaemia–reperfusion injury
-
(IRI). An injury that occurs when blood supply (oxygen) returns to tissue after a period of ischaemia (hypoxia). Damage following oxygen restoration results in inflammation and oxidative damage.
- Connexon
-
A hexamer arrangement of connexins that contains the same or different connexin proteins arranged into an oligomer.
- Gap junctional intercellular communication
-
(GJIC). The process in which contacting cells harbouring functional gap junctions exchange small molecules.
- Perinexus
-
A zone at the periphery of gap junctions that is enriched in connexons and hemichannels, at which Cx43–zonula occludens 1 interactions can occur.
- Connexosomes
-
Double-membraned structures formed from the internalization of gap junction components from contacting cells.
- Intercalated discs
-
Specialized membrane structures at the ends of cardiomyocytes that contain desmosomes, adherens junctions, sodium channels and gap junctions and allow depolarization waves to transmit from one cell to its neighbour.
- Peptide mimetics
-
Short peptide sequences of usually 8–24 amino acids that correspond to segments of connexin proteins that can be used to modulate connexin function.
- Antisense oligodeoxynucleotide
-
(AsODN). A short-chain nucleotide that can be designed to target connexin-encoding RNA to block protein expression.
- Zonula occludens 1
-
(ZO1). ZO1 is a membrane-associated guanylate kinase (MAGUK) scaffolding protein that interacts with Cx43 and tight junction-associated proteins.
Rights and permissions
About this article
Cite this article
Laird, D., Lampe, P. Therapeutic strategies targeting connexins. Nat Rev Drug Discov 17, 905–921 (2018). https://doi.org/10.1038/nrd.2018.138
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2018.138
This article is cited by
-
Simulation of gap junction formation reveals critical role of Cys disulfide redox state in connexin hemichannel docking
Cell Communication and Signaling (2024)
-
Connexin-43 hemichannels orchestrate NOD-like receptor protein-3 (NLRP3) inflammasome activation and sterile inflammation in tubular injury
Cell Communication and Signaling (2023)
-
Genome-wide association study of varicose veins identifies a protective missense variant in GJD3 enriched in the Finnish population
Communications Biology (2023)
-
Cryo-EM structures of human Cx36/GJD2 neuronal gap junction channel
Nature Communications (2023)
-
Tumor suppressor p53 mediates interleukin-6 expression to enable cancer cell evasion of genotoxic stress
Cell Death Discovery (2023)