Key Points
-
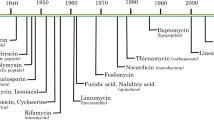
Antibiotics are losing efficacy in treating many bacterial diseases owing to increasing drug resistance. Meanwhile, antibiotic development has lagged; the last new antibiotic class was introduced in 2003.
-
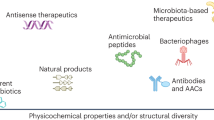
Antivirulence is an alternative approach that focuses on interfering with bacterial virulence factors instead of central growth pathways to treat disease.
-
Antivirulence drugs have been approved by the US Food and Drug Administration for bacterial toxin-mediated diseases, and investigational drugs for antibiotic-resistant bacteria have entered clinical trials. Several others are in preclinical development.
-
Antivirulence drugs are likely to have distinct properties from those of antibiotics, including reduced selection pressure (meaning they are less likely to lead to drug resistance) and minimal perturbation of the healthy microbiota.
-
However, antivirulence strategies pose unique challenges for drug development and clinical use, and such drugs may need to be used in combinations or as adjuncts to antibiotics.
-
Increased investment and effort are needed to develop new virulence inhibitors and advance existing ones through the translational pipeline.
Abstract
The rapid evolution and dissemination of antibiotic resistance among bacterial pathogens are outpacing the development of new antibiotics, but antivirulence agents provide an alternative. These agents can circumvent antibiotic resistance by disarming pathogens of virulence factors that facilitate human disease while leaving bacterial growth pathways — the target of traditional antibiotics — intact. Either as stand-alone medications or together with antibiotics, these drugs are intended to treat bacterial infections in a largely pathogen-specific manner. Notably, development of antivirulence drugs requires an in-depth understanding of the roles that diverse virulence factors have in disease processes. In this Review, we outline the theory behind antivirulence strategies and provide examples of bacterial features that can be targeted by antivirulence approaches. Furthermore, we discuss the recent successes and failures of this paradigm, and new developments that are in the pipeline.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
05 May 2017
In Figure 2, mouse αEbpA serum was incorrectly shown to contain an scFv region. The italicization of bacterial species and genes was also incorrect in some places. These have now been corrected in the online versions of the article.
References
Smith, R. & Coast, J. The true cost of antimicrobial resistance. BMJ 346, f1493 (2013).
Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (CDC, US Department of Health and Human Services, 2013).
Gelband, H. et al. The State of the World's Antibiotics 2015. https://cddep.org/sites/default/files/swa_2015_final.pdf (CDDEP, 2015).
Davies, J. & Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74, 417–433 (2010).
D'Costa, V. M. et al. Antibiotic resistance is ancient. Nature 477, 457–461 (2011).
Hawkey, P. M. & Jones, A. M. The changing epidemiology of resistance. J. Antimicrob. Chemother. 64 (Suppl. 1), i3–i10 (2009).
Pehrsson, E. C. et al. Interconnected microbiomes and resistomes in low-income human habitats. Nature 533, 212–216 (2016).
Liu, Y. Y. et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168 (2016).
Hasman, H. et al. Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, Denmark 2015. Euro Surveill. http://dx.doi.org/10.2807/1560-7917.ES.2015.20.49.30085 (2015).
Sievert, D. M. et al. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin. Infect. Dis. 46, 668–674 (2008).
Kumar, M. Multidrug-resistant Staphylococcus aureus, India, 2013–2015. Emerg. Infect. Dis. 22, 1666–1667 (2016).
Czaplewski, L. et al. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect. Dis. 16, 239–251 (2016).
Clatworthy, A. E., Pierson, E. & Hung, D. T. Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol. 3, 541–548 (2007). This review is the first to focus on the antivirulence paradigm for treating infectious diseases.
Casadevall, A. & Pirofski, L. A. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 67, 3703–3713 (1999).
Allen, R. C., Popat, R., Diggle, S. P. & Brown, S. P. Targeting virulence: can we make evolution-proof drugs? Nat. Rev. Microbiol. 12, 300–308 (2014).
Moeller, A. H. et al. Cospeciation of gut microbiota with hominids. Science 353, 380–382 (2016).
Cho, I. & Blaser, M. J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 13, 260–270 (2012).
Gilmore, M. S. et al. Pheromone killing of multidrug-resistant Enterococcus faecalis V583 by native commensal strains. Proc. Natl Acad. Sci. USA 112, 7273–7278 (2015).
Theriot, C. M. & Young, V. B. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu. Rev. Microbiol. 69, 445–461 (2015).
Keeney, K. M., Yurist-Doutsch, S., Arrieta, M. C. & Finlay, B. B. Effects of antibiotics on human microbiota and subsequent disease. Annu. Rev. Microbiol. 68, 217–235 (2014).
Payne, D. J., Gwynn, M. N., Holmes, D. J. & Pompliano, D. L. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6, 29–40 (2007). This report detailed the difficulties faced by GlaxoSmithKline in developing novel antibiotics in the genomics era.
Ling, L. L. et al. A new antibiotic kills pathogens without detectable resistance. Nature 517, 455–459 (2015).
DiGiandomenico, A. & Sellman, B. R. Antibacterial monoclonal antibodies: the next generation? Curr. Opin. Microbiol. 27, 78–85 (2015).
Alizon, S., Hurford, A., Mideo, N. & Van Baalen, M. Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J. Evol. Biol. 22, 245–259 (2009).
Casadevall, A. & Pirofski, L. Host–pathogen interactions: the attributes of virulence. J. Infect. Dis. 184, 337–344 (2001).
Rice, L. B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197, 1079–1081 (2008). This report identified a cohort of troublesome antibiotic-resistant pathogens and coined the acronym ESKAPE.
Dover, N., Barash, J. R., Hill, K. K., Xie, G. & Arnon, S. S. Molecular characterization of a novel botulinum neurotoxin type H gene. J. Infect. Dis. 209, 192–202 (2014).
Simpson, L. L. Identification of the major steps in botulinum toxin action. Annu. Rev. Pharmacol. Toxicol. 44, 167–193 (2004).
Gill, D. M. Bacterial toxins: a table of lethal amounts. Microbiol. Rev. 46, 86–94 (1982).
Arnon, S. S. et al. Botulinum toxin as a biological weapon: medical and public health management. JAMA 285, 1059–1070 (2001).
Sobel, J., Tucker, N., Sulka, A., McLaughlin, J. & Maslanka, S. Foodborne botulism in the United States, 1990–2000. Emerg. Infect. Dis. 10, 1606–1611 (2004).
Arnon, S. S., Schechter, R., Maslanka, S. E., Jewell, N. P. & Hatheway, C. L. Human botulism immune globulin for the treatment of infant botulism. N. Engl. J. Med. 354, 462–471 (2006). This is the first study that demonstrated efficacy of an antivirulence agent in a randomized clinical trial.
Maslanka, S. E. et al. A novel botulinum neurotoxin, previously reported as serotype H, has a hybrid-like structure with regions of similarity to the structures of serotypes A and F and is neutralized with serotype A antitoxin. J. Infect. Dis. 213, 379–385 (2016).
Holty, J. E. et al. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann. Intern. Med. 144, 270–280 (2006).
Chen, Z., Moayeri, M. & Purcell, R. Monoclonal antibody therapies against anthrax. Toxins (Basel) 3, 1004–1019 (2011).
Nestorovich, E. M. & Bezrukov, S. M. Designing inhibitors of anthrax toxin. Expert Opin. Drug Discov. 9, 299–318 (2014).
Abrami, L. et al. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep. 5, 986–996 (2013).
Migone, T. S. et al. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 361, 135–144 (2009). This study reported the mAb raxibacumab directed against an anthrax virulence factor. Raxibacumab became one of the first FDA-approved anthrax therapeutics.
Greig, S. L. Obiltoxaximab: first global approval. Drugs 76, 823–830 (2016).
Yamamoto, B. J. et al. Obiltoxaximab prevents disseminated Bacillus anthracis infection and improves survival during pre- and post-exposure prophylaxis in animal models of inhalational anthrax. Antimicrob. Agents Chemother. 60, 5796–5805 (2016).
Schneerson, R. et al. Poly(γ-D-glutamic acid) protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: a potential addition to the anthrax vaccine. Proc. Natl Acad. Sci. USA 100, 8945–8950 (2003).
Chen, Z. et al. Pre- and postexposure protection against virulent anthrax infection in mice by humanized monoclonal antibodies to Bacillus anthracis capsule. Proc. Natl Acad. Sci. USA 108, 739–744 (2011).
Kozel, T. R. et al. Protective and immunochemical activities of monoclonal antibodies reactive with the Bacillus anthracis polypeptide capsule. Infect. Immun. 75, 152–163 (2007).
Leffler, D. A. & Lamont, J. T. Clostridium difficile infection. N. Engl. J. Med. 372, 1539–1548 (2015).
Abt, M. C., McKenney, P. T. & Pamer, E. G. Clostridium difficile colitis: pathogenesis and host defence. Nat. Rev. Microbiol. 10, 609–620 (2016).
Lessa, F. C. et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 372, 825–834 (2015).
Akerlund, T. et al. Increased sporulation rate of epidemic Clostridium difficile Type 027/NAP1. J. Clin. Microbiol. 46, 1530–1533 (2008).
Carter, G. P., Rood, J. I. & Lyras, D. The role of toxin A and toxin B in the virulence of Clostridium difficile. Trends Microbiol. 20, 21–29 (2012).
Johnson, S. et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin. Infect. Dis. 59, 345–354 (2014).
Lowy, I. et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J. Med. 362, 197–205 (2010). This study reported the mAb bezlotoxumab directed against a C. difficile toxin. It was later approved by the FDA to reduce recurrence of C. difficile infections.
Bender, K. O. et al. A small-molecule antivirulence agent for treating Clostridium difficile infection. Sci. Transl Med. 7, 306ra148 (2015).
Lynch, E. & Kil, J. Development of ebselen, a glutathione peroxidase mimic, for the prevention and treatment of noise-induced hearing loss. Semin. Hear. 30, 47–55 (2009).
Gustafsson, T. N. et al. Ebselen and analogs as inhibitors of Bacillus anthracis thioredoxin reductase and bactericidal antibacterials targeting Bacillus species. Staphylococcus aureus and Mycobacterium tuberculosis. Biochim. Biophys. Acta 1860, 1265–1271 (2016).
Thangamani, S., Younis, W. & Seleem, M. N. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci. Rep. 5, 11596 (2015).
Darkoh, C., Odo, C. & DuPont, H. L. Accessory gene regulator-1 locus is essential for virulence and pathogenesis of Clostridium difficile. mBio 7, e01237-16 (2016).
Martin, M. J. et al. The agr locus regulates virulence and colonization genes in Clostridium difficile 027. J. Bacteriol. 195, 3672–3681 (2013).
Dantes, R. et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern. Med. 173, 1970–1978 (2013).
Otto, M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu. Rev. Microbiol. 64, 143–162 (2010).
Li, M. et al. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat. Med. 18, 816–819 (2012).
Chen, Y., Chatterjee, S. S., Porcella, S. F., Yu, Y. S. & Otto, M. Complete genome sequence of a Panton-Valentine leukocidin-negative community-associated methicillin-resistant Staphylococcus aureus strain of sequence type 72 from Korea. PLoS ONE 8, e72803 (2013).
Li, M. et al. Virulence determinants associated with the Asian community-associated methicillin-resistant Staphylococcus aureus lineage ST59. Sci. Rep. 6, 27899 (2016).
Moran, G. J. et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355, 666–674 (2006).
Bhakdi, S. & Tranum-Jensen, J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 55, 733–751 (1991).
Oganesyan, V. et al. Mechanisms of neutralization of a human anti-alpha-toxin antibody. J. Biol. Chem. 289, 29874–29880 (2014).
Sharma-Kuinkel, B. K. et al. Characterization of alpha-toxin hla gene variants, alpha-toxin expression levels, and levels of antibody to alpha-toxin in hemodialysis and postsurgical patients with Staphylococcus aureus bacteremia. J. Clin. Microbiol. 53, 227–236 (2015).
Rouha, H. et al. Five birds, one stone: neutralization of alpha-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. mAbs 7, 243–254 (2015). This study reported a single mAb that cross-reacted with five different S. aureus toxins that share a conserved epitope.
Badarau, A. et al. Context matters: the importance of dimerization-induced conformation of the LukGH leukocidin of Staphylococcus aureus for the generation of neutralizing antibodies. mAbs 8, 1347–1360 (2016).
Vandenesch, F., Lina, G. & Henry, T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front. Cell. Infect. Microbiol. 2, 12 (2012).
Schneewind, O., Model, P. & Fischetti, V. A. Sorting of protein A to the staphylococcal cell wall. Cell 70, 267–281 (1992).
Mazmanian, S. K., Liu, G., Jensen, E. R., Lenoy, E. & Schneewind, O. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl Acad. Sci. USA 97, 5510–5515 (2000).
Zhang, J. et al. Antiinfective therapy with a small molecule inhibitor of Staphylococcus aureus sortase. Proc. Natl Acad. Sci. USA 111, 13517–13522 (2014).
Ji, G., Beavis, R. & Novick, R. P. Bacterial interference caused by autoinducing peptide variants. Science 276, 2027–2030 (1997).
Jarraud, S. et al. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J. Bacteriol. 182, 6517–6522 (2000).
Sully, E. K. et al. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog. 10, e1004174 (2014).
Smith, R. S. & Iglewski, B. H. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 6, 56–60 (2003).
Deziel, E. et al. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol. Microbiol. 55, 998–1014 (2005).
Lee, J. et al. A cell-cell communication signal integrates quorum sensing and stress response. Nat. Chem. Biol. 9, 339–343 (2013).
Wagner, S. et al. Novel strategies for the treatment of Pseudomonas aeruginosa infections. J. Med. Chem. 59, 5929–5969 (2016).
Zhu, J. & Kaufmann, G. F. Quo vadis quorum quenching? Curr. Opin. Pharmacol. 13, 688–698 (2013).
Tateda, K. et al. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45, 1930–1933 (2001).
Kai, T. et al. A low concentration of azithromycin inhibits the mRNA expression of N-acyl homoserine lactone synthesis enzymes, upstream of lasI or rhlI, in Pseudomonas aeruginosa. Pulm. Pharmacol. Ther. 22, 483–486 (2009).
Kohler, T., Perron, G. G., Buckling, A. & van Delden, C. Quorum sensing inhibition selects for virulence and cooperation in Pseudomonas aeruginosa. PLoS Pathog. 6, e1000883 (2010).
Garcia-Contreras, R. et al. Resistance to the quorum-quenching compounds brominated furanone C-30 and 5-fluorouracil in Pseudomonas aeruginosa clinical isolates. Pathog. Dis. 68, 8–11 (2013).
Smith, E. E. et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl Acad. Sci. USA 103, 8487–8492 (2006).
Marvig, R. L., Sommer, L. M., Molin, S. & Johansen, H. K. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat. Genet. 47, 57–64 (2015).
Starkey, M. et al. Identification of anti-virulence compounds that disrupt quorum-sensing regulated acute and persistent pathogenicity. PLoS Pathog. 10, e1004321 (2014).
DiGiandomenico, A. et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J. Exp. Med. 209, 1273–1287 (2012).
Warrener, P. et al. A novel anti-PcrV antibody providing enhanced protection against Pseudomonas aeruginosa in multiple animal infection models. Antimicrob. Agents Chemother. 58, 4384–4391 (2014).
DiGiandomenico, A. et al. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl Med. 6, 262ra155 (2014). This study reported a novel bispecific antibody derivative that targeted two virulence factors simultaneously with improved efficacy in animal models.
Kaper, J. B., Nataro, J. P. & Mobley, H. L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 (2004).
McGann, P. et al. Escherichia coli harboring mcr-1 and blaCTX-M on a novel IncF plasmid: first report of mcr-1 in the United States. Antimicrob. Agents Chemother. 60, 4420–4421 (2016).
Castanheira, M. et al. Detection of mcr-1 among Escherichia coli clinical isolates collected worldwide as part of the SENTRY antimicrobial surveillance program in 2014 and 2015. Antimicrob. Agents Chemother. 60, 5623–5624 (2016).
Mediavilla, J. R. et al. Colistin- and carbapenem-resistant Escherichia coli harboring mcr-1 and blaNDM-5, causing a complicated urinary tract infection in a patient from the United States. mBio 7, e01191-16 (2016).
Goren, M. G. et al. Transfer of carbapenem-resistant plasmid from Klebsiella pneumoniae ST258 to Escherichia coli in patient. Emerg. Infect. Dis. 16, 1014–1017 (2010).
Cremet, L. et al. Nosocomial outbreak of carbapenem-resistant Enterobacter cloacae highlighting the interspecies transferability of the blaOXA-48 gene in the gut flora. J. Antimicrob. Chemother. 67, 1041–1043 (2012).
Obrig, T. G. et al. Endothelial heterogeneity in Shiga toxin receptors and responses. J. Biol. Chem. 268, 15484–15488 (1993).
Wong, C. S. et al. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: a multivariable analysis. Clin. Infect. Dis. 55, 33–41 (2012).
Zhang, X. et al. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J. Infect. Dis. 181, 664–670 (2000).
Walterspiel, J. N., Ashkenazi, S., Morrow, A. L. & Cleary, T. G. Effect of subinhibitory concentrations of antibiotics on extracellular Shiga-like toxin I. Infection 20, 25–29 (1992).
Takeda, T., Yoshino, K., Adachi, E., Sato, Y. & Yamagata, K. In vitro assessment of a chemically synthesized Shiga toxin receptor analog attached to chromosorb P (Synsorb Pk) as a specific absorbing agent of Shiga toxin 1 and 2. Microbiol. Immunol. 43, 331–337 (1999).
Armstrong, G. D. et al. A phase I study of chemically synthesized verotoxin (Shiga-like toxin) Pk-trisaccharide receptors attached to chromosorb for preventing hemolytic-uremic syndrome. J. Infect. Dis. 171, 1042–1045 (1995).
Trachtman, H. et al. Effect of an oral Shiga toxin-binding agent on diarrhea-associated hemolytic uremic syndrome in children: a randomized controlled trial. JAMA 290, 1337–1344 (2003).
Yamagami, S. et al. Efficacy of postinfection treatment with anti-Shiga toxin (Stx) 2 humanized monoclonal antibody TMA-15 in mice lethally challenged with Stx-producing Escherichia coli. J. Infect. Dis. 184, 738–742 (2001).
Sauter, K. A. et al. Mouse model of hemolytic-uremic syndrome caused by endotoxin-free Shiga toxin 2 (Stx2) and protection from lethal outcome by anti-Stx2 antibody. Infect. Immun. 76, 4469–4478 (2008).
Lopez, E. L. et al. Safety and pharmacokinetics of urtoxazumab, a humanized monoclonal antibody, against Shiga-like toxin 2 in healthy adults and in pediatric patients infected with Shiga-like toxin-producing Escherichia coli. Antimicrob. Agents Chemother. 54, 239–243 (2010).
Bitzan, M. et al. Safety and pharmacokinetics of chimeric anti-Shiga toxin 1 and anti-Shiga toxin 2 monoclonal antibodies in healthy volunteers. Antimicrob. Agents Chemother. 53, 3081–3087 (2009).
Flores-Mireles, A. L., Walker, J. N., Caparon, M. & Hultgren, S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284 (2015).
Al-Hasan, M. N., Eckel-Passow, J. E. & Baddour, L. M. Bacteremia complicating gram-negative urinary tract infections: a population-based study. J. Infect. 60, 278–285 (2010).
Han, Z. et al. Structure-based drug design and optimization of mannoside bacterial FimH antagonists. J. Med. Chem. 53, 4779–4792 (2010).
Jarvis, C. et al. Antivirulence isoquinolone mannosides: optimization of the biaryl aglycone for FimH lectin binding affinity and efficacy in the treatment of chronic UTI. ChemMedChem 11, 367–373 (2016).
Greene, S. E. et al. Pilicide ec240 disrupts virulence circuits in uropathogenic Escherichia coli. mBio 5, e02038 (2014).
Chorell, E. et al. Design and synthesis of C-2 substituted thiazolo and dihydrothiazolo ring-fused 2-pyridones: pilicides with increased antivirulence activity. J. Med. Chem. 53, 5690–5695 (2010).
Cusumano, C. K. et al. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci. Transl Med. 3, 109ra115 (2011).
Totsika, M. et al. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J. Infect. Dis. 208, 921–928 (2013).
Kisiela, D. I. et al. Inhibition and reversal of microbial attachment by an antibody with parasteric activity against the FimH adhesin of uropathogenic E. coli. PLoS Pathog. 11, e1004857 (2015).
Wang, Q. et al. Target-agnostic identification of functional monoclonal antibodies against Klebsiella pneumoniae multimeric MrkA fimbrial subunit. J. Infect. Dis. 213, 1800–1808 (2016). This report applied a phenotypic screen and phage display to identify mAbs and scFvs that target a surface-associated virulence factor.
Yu, V. L. et al. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg. Infect. Dis. 13, 986–993 (2007).
Lin, J. C. et al. High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess. Microbes Infect. 6, 1191–1198 (2004).
Sievert, D. M. et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 34, 1–14 (2013).
Oikonomou, O. et al. Rapid dissemination of colistin and carbapenem resistant Acinetobacter baumannii in Central Greece: mechanisms of resistance, molecular identification and epidemiological data. BMC Infect. Dis. 15, 559 (2015).
Qureshi, Z. A. et al. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin. Infect. Dis. 60, 1295–1303 (2015).
Mavroidi, A. et al. Molecular identification of tigecycline- and colistin-resistant carbapenemase-producing Acinetobacter baumannii from a Greek hospital from 2011 to 2013. J. Med. Microbiol. 64, 993–997 (2015).
European Antimicrobial Resistance Surveillance Network. Antimicrobial resistance surveillance in Europe. http://ecdc.europa.eu/en/publications/publications/antimicrobial-resistance-europe-2014.pdf (EARS-Net, 2014).
Lin, L. et al. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. mBio 3, e00312-12 (2012).
Moffatt, J. H. et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 54, 4971–4977 (2010).
Garcia-Quintanilla, M. et al. Inhibition of LpxC increases antibiotic susceptibility in Acinetobacter baumannii. Antimicrob. Agents Chemother. 60, 5076–5079 (2016).
Niu, C., Clemmer, K. M., Bonomo, R. A. & Rather, P. N. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J. Bacteriol. 190, 3386–3392 (2008).
Bhargava, N., Sharma, P. & Capalash, N. Quorum sensing in Acinetobacter: an emerging pathogen. Crit. Rev. Microbiol. 36, 349–360 (2010).
Stacy, D. M., Welsh, M. A., Rather, P. N. & Blackwell, H. E. Attenuation of quorum sensing in the pathogen Acinetobacter baumannii using non-native N-Acyl homoserine lactones. ACS Chem. Biol. 7, 1719–1728 (2012).
Hung, D. T., Shakhnovich, E. A., Pierson, E. & Mekalanos, J. J. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science 310, 670–674 (2005).
Nait Chabane, Y. et al. Virstatin inhibits biofilm formation and motility of Acinetobacter baumannii. BMC Microbiol. 14, 62 (2014).
Oh, M. H. & Choi, C. H. Role of LuxIR homologue AnoIR in Acinetobacter nosocomialis and the effect of virstatin on the expression of anoR gene. J. Microbiol. Biotechnol. 25, 1390–1400 (2015).
Thompson, M. G., Corey, B. W., Si, Y., Craft, D. W. & Zurawski, D. V. Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob. Agents Chemother. 56, 5419–5421 (2012).
de Leseleuc, L., Harris, G., KuoLee, R. & Chen, W. In vitro and in vivo biological activities of iron chelators and gallium nitrate against Acinetobacter baumannii. Antimicrob. Agents Chemother. 56, 5397–5400 (2012).
Wang, N., Ozer, E. A., Mandel, M. J. & Hauser, A. R. Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. mBio 5, e01163-14 (2014).
Subashchandrabose, S. et al. Acinetobacter baumannii genes required for bacterial survival during bloodstream infection. mSphere 1, e00013-15 (2016).
Weber, B. S., Harding, C. M. & Feldman, M. F. Pathogenic Acinetobacter: from the cell surface to infinity and beyond. J. Bacteriol. 198, 880–887 (2016).
Flores-Mireles, A. L. et al. Antibody-based therapy for Enterococcal catheter-associated urinary tract infections. mBio 7, e01653-16 (2016).
Van Tyne, D., Martin, M. J. & Gilmore, M. S. Structure, function, and biology of the Enterococcus faecalis cytolysin. Toxins (Basel) 5, 895–911 (2013).
Huycke, M. M., Spiegel, C. A. & Gilmore, M. S. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 35, 1626–1634 (1991).
Desouky, S. E. et al. Cyclodepsipeptides produced by actinomycetes inhibit cyclic-peptide-mediated quorum sensing in Gram-positive bacteria. FEMS Microbiol. Lett. 362, fnv109 (2015).
Nakayama, J. et al. Development of a peptide antagonist against fsr quorum sensing of Enterococcus faecalis. ACS Chem. Biol. 8, 804–811 (2013).
Nakayama, J. et al. Siamycin attenuates fsr quorum sensing mediated by a gelatinase biosynthesis-activating pheromone in Enterococcus faecalis. J. Bacteriol. 189, 1358–1365 (2007).
Klipstein, F. A. & Engert, R. F. Partial purification and properties of Enterobacter cloacae heat-stable enterotoxin. Infect. Immun. 13, 1307–1314 (1976).
Barnes, A. I., Paraje, M. G., Battan, P. del C. & Albesa, I. Molecular properties and metabolic effect on blood cells produced by a new toxin of Enterobacter cloacae. Cell Biol. Toxicol. 17, 409–418 (2001)
Barnes, A. I., Ortiz, C., Paraje, M. G., Balanzino, L. E. & Albesa, I. Purification and characterization of a cytotoxin from Enterobacter cloacae. Can. J. Microbiol. 43, 729–733 (1997).
Paton, A. W. & Paton, J. C. Enterobacter cloacae producing a Shiga-like toxin II-related cytotoxin associated with a case of hemolytic-uremic syndrome. J. Clin. Microbiol. 34, 463–465 (1996).
Probert, W. S., McQuaid, C. & Schrader, K. Isolation and identification of an Enterobacter cloacae strain producing a novel subtype of Shiga toxin type 1. J. Clin. Microbiol. 52, 2346–2351 (2014).
Herold, S., Karch, H. & Schmidt, H. Shiga toxin-encoding bacteriophages — genomes in motion. Int. J. Med. Microbiol. 294, 115–121 (2004).
Khalil, R. K., Skinner, C., Patfield, S. & He, X. Phage-mediated Shiga toxin (Stx) horizontal gene transfer and expression in non-Shiga toxigenic Enterobacter and Escherichia coli strains. Pathog. Dis. 74, ftw037 (2016).
Van Tiel-Menkveld, G. J., Mentjox-Vervuurt, J. M., Oudega, B. & de Graaf, F. K. Siderophore production by Enterobacter cloacae and a common receptor protein for the uptake of aerobactin and cloacin DF13. J. Bacteriol. 150, 490–497 (1982).
Kim, S. M. et al. Involvement of curli fimbriae in the biofilm formation of Enterobacter cloacae. J. Microbiol. 50, 175–178 (2012).
Zogaj, X., Bokranz, W., Nimtz, M. & Romling, U. Production of cellulose and curli fimbriae by members of the family Enterobacteriaceae isolated from the human gastrointestinal tract. Infect. Immun. 71, 4151–4158 (2003).
Krzyminska, S., Mokracka, J., Koczura, R. & Kaznowski, A. Cytotoxic activity of Enterobacter cloacae human isolates. FEMS Immunol. Med. Microbiol. 56, 248–252 (2009).
Rezzonico, F., Smits, T. H. & Duffy, B. Detection of AI-2 receptors in genomes of Enterobacteriaceae suggests a role of type-2 quorum sensing in closed ecosystems. Sensors (Basel) 12, 6645–6665 (2012).
Skinner, C., Patfield, S., Khalil, R., Kong, Q. & He, X. New monoclonal antibodies against a novel subtype of Shiga toxin 1 produced by Enterobacter cloacae and their use in analysis of human serum. mSphere 1, e00099-15 (2016).
World Health Organization. Global Tuberculosis Report 2015. http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1 (WHO, 2015).
Kaufmann, S. H. et al. Progress in tuberculosis vaccine development and host-directed therapies — a state of the art review. Lancet Respir. Med. 2, 301–320 (2014).
Colditz, G. A. et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271, 698–702 (1994).
Brown, R. M. et al. Lipoarabinomannan-reactive human secretory immunoglobulin A responses induced by mucosal bacille Calmette-Guerin vaccination. J. Infect. Dis. 187, 513–517 (2003).
Beyazova, U., Rota, S., Cevheroglu, C. & Karsligil, T. Humoral immune response in infants after BCG vaccination. Tuber. Lung Dis. 76, 248–253 (1995).
Steingart, K. R. et al. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin. Vaccine Immunol. 16, 260–276 (2009).
Grosset, J. Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrob. Agents Chemother. 47, 833–836 (2003).
Kang, B. K. & Schlesinger, L. S. Characterization of mannose receptor-dependent phagocytosis mediated by Mycobacterium tuberculosis lipoarabinomannan. Infect. Immun. 66, 2769–2777 (1998).
Menozzi, F. D. et al. Identification of a heparin-binding hemagglutinin present in mycobacteria. J. Exp. Med. 184, 993–1001 (1996).
Yuan, Y., Crane, D. D. & Barry, C. E. III. Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial alpha-crystallin homolog. J. Bacteriol. 178, 4484–4492 (1996).
Hamasur, B. et al. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab') fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin. Exp. Immunol. 138, 30–38 (2004).
Balu, S. et al. A novel human IgA monoclonal antibody protects against tuberculosis. J. Immunol. 186, 3113–3119 (2011).
Pethe, K. et al. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 412, 190–194 (2001).
Sun, J. et al. The tuberculosis necrotizing toxin kills macrophages by hydrolyzing NAD. Nat. Struct. Mol. Biol. 22, 672–678 (2015).
Groschel, M. I., Sayes, F., Simeone, R., Majlessi, L. & Brosch, R. ESX secretion systems: mycobacterial evolution to counter host immunity. Nat. Rev. Microbiol. 14, 677–691 (2016).
Boucher, H. W. et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48, 1–12 (2009).
Waters, C. M. & Bassler, B. L. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346 (2005).
Papenfort, K. & Bassler, B. L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 14, 576–588 (2016).
Rutherford, S. T. & Bassler, B. L. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2, a012427 (2012).
Kong, K. F., Vuong, C. & Otto, M. Staphylococcus quorum sensing in biofilm formation and infection. Int. J. Med. Microbiol. 296, 133–139 (2006).
Fowler, V. G. Jr et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J. Infect. Dis. 190, 1140–1149 (2004).
Garcia-Contreras, R., Maeda, T. & Wood, T. K. Can resistance against quorum-sensing interference be selected? ISME J. 10, 4–10 (2016).
Gerdt, J. P. & Blackwell, H. E. Competition studies confirm two major barriers that can preclude the spread of resistance to quorum-sensing inhibitors in bacteria. ACS Chem. Biol. 9, 2291–2299 (2014).
Mellbye, B. & Schuster, M. The sociomicrobiology of antivirulence drug resistance: a proof of concept. mBio 2, e00131-11 (2011).
Carnes, E. C. et al. Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nat. Chem. Biol. 6, 41–45 (2010).
Maeda, T. et al. Quorum quenching quandary: resistance to antivirulence compounds. ISME J. 6, 493–501 (2012).
Manefield, M. et al. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148, 1119–1127 (2002).
Babcock, G. J. et al. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect. Immun. 74, 6339–6347 (2006).
Kaufmann, G. F. et al. Antibody interference with N-acyl homoserine lactone-mediated bacterial quorum sensing. J. Am. Chem. Soc. 128, 2802–2803 (2006).
Baer, M. et al. An engineered human antibody fab fragment specific for Pseudomonas aeruginosa PcrV antigen has potent antibacterial activity. Infect. Immun. 77, 1083–1090 (2009).
Anantharajah, A. et al. Inhibition of the injectisome and flagellar type III secretion systems by INP1855 impairs Pseudomonas aeruginosa pathogenicity and inflammasome activation. J. Infect. Dis. 214, 1105–1116 (2016).
Williams, J. D. et al. Synthesis and structure-activity relationships of novel phenoxyacetamide inhibitors of the Pseudomonas aeruginosa type III secretion system (T3SS). Bioorg. Med. Chem. 23, 1027–1043 (2015).
Bowlin, N. O. et al. Mutations in the Pseudomonas aeruginosa needle protein gene pscF confer resistance to phenoxyacetamide inhibitors of the type III secretion system. Antimicrob. Agents Chemother. 58, 2211–2220 (2014).
Eibergen, N. R., Moore, J. D., Mattmann, M. E. & Blackwell, H. E. Potent and selective modulation of the RhlR quorum sensing receptor by using non-native ligands: an emerging target for virulence control in Pseudomonas aeruginosa. Chembiochem 16, 2348–2356 (2015).
O'Loughlin, C. T. et al. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl Acad. Sci. USA 110, 17981–17986 (2013).
Amara, N. et al. Covalent inhibition of bacterial quorum sensing. J. Am. Chem. Soc. 131, 10610–10619 (2009).
Hentzer, M. et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22, 3803–3815 (2003).
Wu, H. et al. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J. Antimicrob. Chemother. 53, 1054–1061 (2004).
Smith, K. M., Bu, Y. & Suga, H. Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem. Biol. 10, 81–89 (2003).
Acknowledgements
The authors are supported by the Intramural Research Program of the US National Institute of Allergy and Infectious Diseases and the Postdoctoral Research Associate Program of the US National Institute of General Medical Sciences, US National Institutes of Health, Bethesda, Maryland, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
DATABASES
FURTHER INFORMATION
Glossary
- Horizontal gene transfer
-
The exchange of genetic information between organisms in a manner other than by traditional reproduction. Horizontal gene transfer is a key mechanism for the evolution of antibiotic resistance in bacteria.
- Virulence factors
-
A broad term used to define molecules produced by pathogens that promote disease or damage the host. They include, but are not limited to, adhesins, regulators, toxins and siderophores.
- Microbiota
-
Collective term for all microflora that are found residing in a given environment. Many microorganisms are considered to be part of the beneficial microbiota on human skin and gut.
- Bacterial toxins
-
A diverse variety of molecules that are produced by many pathogenic bacteria and cause injury to cells. Toxins may directly form pores in eukaryotic membranes or transfer enzymes that deregulate essential intracellular pathways or induce cell death. In addition, some toxins modulate immune cell function to thwart immune responses and facilitate immune evasion for the invading pathogens.
- Empiric therapy
-
The use of broad-spectrum antimicrobial therapy before a definitive diagnosis of the disease-causing organism.
- Adhesins
-
Cell-surface components or appendages of bacteria that facilitate adhesion to other cells or to surfaces: for example, fimbriae (pili). Adhesins contribute to host specificity and tissue tropism.
- Siderophores
-
Low-molecular-weight, high-affinity metal chelating agents produced by microorganisms under low-nutrient conditions. Bacteria depend on siderophores to acquire iron in vertebrate hosts so they can replicate and cause disease.
- Biofilm
-
A surface-attached agglomeration of microorganisms embedded in an extracellular matrix. Biofilm-associated infections are difficult to eradicate and are an important reservoir for disseminating and recurring serious infections.
- Quorum sensing
-
A phenomenon describing the regulation of gene expression in response to cell population density and the production and secretion of signalling molecules (auto-inducers) by quorum-sensing bacteria. Effector functions of quorum sensing include the regulation of genes involved in virulence and biofilm formation.
- Fab and F(ab′)2
-
The antigen-binding fragment of an immunoglobulin G antibody after proteolytic digestion. Fab and F(ab′)2 are monovalent and divalent, respectively, and are produced under differing digestion conditions.
- Monoclonal antibodies
-
(mAbs). Antibodies produced by a single B cell clone that are specific to one epitope of an antigen.
- Polyclonal antibodies
-
A pool of antibodies from different B cells that recognize multiple epitopes on the same antigen.
- Response regulator
-
One part of a two-component system — comprising a membrane-embedded sensor and the cytoplasmic response regulator — that activates transcription of a specific set of genes in response to certain stimuli.
- Type III secretion system
-
(T3SS). A needle-like apparatus found in Gram-negative bacteria that delivers substrates across the inner and outer bacterial membranes and the eukaryotic membranes to the host membrane or cytosol.
- Single-chain variable fragment
-
(scFv). An antibody derivative in which the variable domains from the heavy and light chains have been fused with a linker. An scFv retains the capability to bind the target antigen.
- Pan-drug-resistant
-
Resistant to all approved antimicrobial agents with activity against a specific bacterial species.
- Type VII secretion system
-
A specialized secretion system found in Gram-positive bacteria, the most studied of which are in Mycobacterium tuberculosis. Protein substrates are first exported across the inner membrane by an ATP-dependent multimeric protein complex. Secreted substrates can localize to the culture supernatant or remain embedded in the highly hydrophobic mycobacterial cell envelope. Homologous systems are found in other bacteria from the phyla Actinobacteria and Firmicutes.
Rights and permissions
About this article
Cite this article
Dickey, S., Cheung, G. & Otto, M. Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discov 16, 457–471 (2017). https://doi.org/10.1038/nrd.2017.23
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2017.23
This article is cited by
-
Nanotechnology’s frontier in combatting infectious and inflammatory diseases: prevention and treatment
Signal Transduction and Targeted Therapy (2024)
-
Ziziphus spina-christi (L.) Willd. Leaves Extract Affecting agr Quorum Sensing System in Staphylococcus aureus
Arabian Journal for Science and Engineering (2024)
-
Quercetin: a promising virulence inhibitor of Pseudomonas aeruginosa LasB in vitro
Applied Microbiology and Biotechnology (2024)
-
Unveiling the hidden language of bacteria: anti-quorum sensing strategies for gram-negative bacteria infection control
Archives of Microbiology (2024)
-
Antimicrobial potential of myricetin-coated zinc oxide nanocomposite against drug-resistant Clostridium perfringens
BMC Microbiology (2023)