Key Points
-
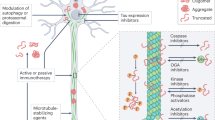
Aggregates of the microtubule-associated protein tau are a defining feature of a group of neurodegenerative disorders collectively known as tauopathies and are a hallmark lesion of Alzheimer disease.
-
Tau exists as six major isoforms in the adult human brain and is subject to massive post-translational modifications, including phosphorylation, acetylation, ubiquitylation and truncation.
-
The advent of novel biomarkers such as PET tracers and the lower regulatory hurdles for treating rare forms of tauopathy, such as progressive supranuclear palsy, have facilitated clinical trials targeting tauopathies.
-
Drug development has also been facilitated by transgenic animal models and by better insights into the physiological and pathological roles of tau and its different isoforms.
-
An interesting new thread has been added to the field with the hypothesis that tau pathology propagates extracellularly.
-
Some challenges faced in the treatment of tauopathies are specific to tau, whereas others, such as the presence of the blood–brain barrier, represent a general challenge in the treatment of diseases of the brain.
-
Clinical trials targeting tau have included more than a dozen diverse (and not yet exhausted) strategies in recent years.
Abstract
Aggregates of the microtubule-associated protein tau are a defining feature of several neurodegenerative diseases that are collectively known as tauopathies, and constitute one of the hallmark lesions of Alzheimer disease (AD). Given the lack of efficacy to date of amyloid-β-targeted therapies for AD, interest is growing in tau as a potential alternative target. Several drug candidates, which are now in clinical trials, aim to reduce tau levels or to prevent the aggregation or pathological post-translation modifications of this protein. In this Review, we discuss preclinical and clinical studies in light of an increased understanding of the physiological and pathological roles of tau, advances in animal models of tauopathy, the identification of novel targets and the availability of novel tracers to track tau.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brier, M. R. et al. Tau and Abeta imaging, CSF measures, and cognition in Alzheimer's disease. Sci. Transl Med. 8, 338ra366 (2016).
Ikonomovic, M. D. et al. Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp. Neurol. 190, 192–203 (2004).
Ma, Y. et al. Increasing the efficiency of CRISPR/Cas9-mediated precise genome editing in rats by inhibiting NHEJ and using Cas9 protein. RNA Biol. 13, 605–612 (2016).
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016). This study established a miniaturized bioreactor for human iPS cell-derived 3D forebrain organoid cultures that recapitulates key features of human cortical development, enabling both a quantitative analysis for modelling human brain development and therapeutic drug screening.
Goedert, M., Spillantini, M. G., Jakes, R., Rutherford, D. & Crowther, R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3, 519–526 (1989).
Arendt, T., Stieler, J. T. & Holzer, M. Tau and tauopathies. Brain Res. Bull. 126, 238–292 (2016).
Goedert, M. et al. PTL-1, a microtubule-associated protein with tau-like repeats from the nematode Caenorhabditis elegans. J. Cell Sci. 109, 2661–2672 (1996).
McDermott, J. B., Aamodt, S. & Aamodt, E. Ptl-1, a Caenorhabditis elegans gene whose products are homologous to the tau microtubule-associated proteins. Biochemistry 35, 9415–9423 (1996).
Chew, Y. L., Fan, X., Götz, J. & Nicholas, H. R. Protein with tau-like repeats regulates neuronal integrity and lifespan in C. elegans. J. Cell Sci. 126, 2079–2091 (2013).
Ittner, L. M. et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell 142, 387–397 (2010).
Zempel, H., Thies, E., Mandelkow, E. & Mandelkow, E. M. Aβ oligomers cause localized Ca2+ elevation, missorting of endogenous tau into dendrites, tau phosphorylation, and destruction of microtubules and spines. J. Neurosci. 30, 11938–11950 (2010).
Sultan, A. et al. Nuclear tau, a key player in neuronal DNA protection. J. Biol. Chem. 286, 4566–4575 (2011).
Gunawardana, C. G. et al. The Human tau interactome: binding to the ribonucleoproteome, and impaired binding of the proline-to-leucine mutant at position 301 (P301L) to chaperones and the proteasome. Mol. Cell Proteomics 14, 3000–3014 (2015).
Multhaup, G., Huber, O., Buee, L. & Galas, M. C. Amyloid precursor protein (APP) metabolites APP intracellular fragment (AICD), Aβ42, and tau in nuclear roles. J. Biol. Chem. 290, 23515–23522 (2015).
Gauthier-Kemper, A. et al. The frontotemporal dementia mutation R406W blocks tau's interaction with the membrane in an annexin A2-dependent manner. J. Cell Biol. 192, 647–661 (2011).
Lee, G. et al. Phosphorylation of tau by fyn: implications for Alzheimer's disease. J. Neurosci. 24, 2304–2312 (2004).
Abraha, A. et al. C-Terminal inhibition of tau assembly in vitro and in Alzheimer's disease. J. Cell Sci. 113, 3737–3745 (2000).
Flores-Rodriguez, P. et al. The relationship between truncation and phosphorylation at the C-terminus of tau protein in the paired helical filaments of Alzheimer's disease. Front. Neurosci. 9, 33 (2015).
Khurana, V. et al. Lysosomal dysfunction promotes cleavage and neurotoxicity of tau in vivo. PLoS Genet. 6, e1001026 (2010).
Liu, C., Song, X., Nisbet, R. & Götz, J. Co-immunoprecipitation with tau isoform-specific antibodies reveals distinct protein interactions, and highlights a putative role for 2N tau in disease. J. Biol. Chem. 291, 161–174 (2016).
Regan, P. et al. Tau phosphorylation at serine 396 residue is required for hippocampal LTD. J. Neurosci. 35, 4804–4812 (2015).
Frandemiche, M. L. et al. Activity-dependent tau protein translocation to excitatory synapse is disrupted by exposure to amyloid-β oligomers. J. Neurosci. 34, 6084–6097 (2014).
Mandell, J. W. & Banker, G. A. A spatial gradient of tau protein phosphorylation in nascent axons. J. Neurosci. 16, 5727–5740 (1996).
Dixit, R., Ross, J. L., Goldman, Y. E. & Holzbaur, E. L. Differential regulation of dynein and kinesin motor proteins by tau. Science 319, 1086–1089 (2008).
Morris, M. et al. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat. Neurosci. 18, 1183–1189 (2015).
Kopke, E. et al. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J. Biol. Chem. 268, 24374–24384 (1993).
Chen, F., David, D., Ferrari, A. & Götz, J. Posttranslational modifications of tau — role in human tauopathies and modeling in transgenic animals. Curr. Drug Targets 5, 503–515 (2004).
Braak, H., Thal, D. R., Ghebremedhin, E. & Del Tredici, K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 70, 960–969 (2011).
Elbaum-Garfinkle, S. & Rhoades, E. Identification of an aggregation-prone structure of tau. J. Am. Chem. Soc. 134, 16607–16613 (2012).
Li, X. et al. Novel diffusion barrier for axonal retention of Tau in neurons and its failure in neurodegeneration. EMBO J. 30, 4825–4837 (2011).
Braak, E., Braak, H. & Mandelkow, E. M. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. 87, 554–567 (1994).
Liu, F., Grundke-Iqbal, I., Iqbal, K. & Gong, C. X. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 22, 1942–1950 (2005).
Steinhilb, M. L., Dias-Santagata, D., Fulga, T. A., Felch, D. L. & Feany, M. B. Tau phosphorylation sites work in concert to promote neurotoxicity in vivo. Mol. Biol. Cell 18, 5060–5068 (2007).
Irwin, D. J. et al. Acetylated tau, a novel pathological signature in Alzheimer's disease and other tauopathies. Brain 135, 807–818 (2012).
Cohen, T. J. et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2, 252 (2011).
Min, S. W. et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 21, 1154–1162 (2015). This paper demonstrates that tau acetylation at a single site identified in the AD brain induces tauopathy and cognitive deficits in vivo , highlighting a role for acetylation in tauopathy.
Thomas, S. N. et al. Dual modification of Alzheimer's disease PHF-tau protein by lysine methylation and ubiquitylation: a mass spectrometry approach. Acta Neuropathol. 123, 105–117 (2012).
Wei, Y. et al. Ribosylation triggering Alzheimer's disease-like tau hyperphosphorylation via activation of CaMKII. Aging Cell 14, 754–763 (2015).
Reyes, J. F., Fu, Y., Vana, L., Kanaan, N. M. & Binder, L. I. Tyrosine nitration within the proline-rich region of tau in Alzheimer's disease. Am. J. Pathol. 178, 2275–2285 (2011).
Zhang, Z. et al. Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer's disease. Nat. Med. 20, 1254–1262 (2014). This study identified asparagine endopeptidase-mediated tau truncation as a crucial event in tau-mediated neurodegeneration.
Crary, J. F. et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 128, 755–766 (2014).
Chare, L. et al. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J. Neurol. Neurosurg. Psychiatry 85, 865–870 (2014).
Taniguchi-Watanabe, S. et al. Biochemical classification of tauopathies by immunoblot, protein sequence and mass spectrometric analyses of sarkosyl-insoluble and trypsin-resistant tau. Acta Neuropathol. 131, 267–280 (2016).
Ittner, L. M. et al. Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proc. Natl Acad. Sci. USA 105, 15997–16002 (2008).
Ittner, L. M., Ke, Y. D. & Götz, J. Phosphorylated tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. J. Biol. Chem. 284, 20909–20916 (2009).
Gerson, J. E., Castillo-Carranza, D. L. & Kayed, R. Advances in therapeutics for neurodegenerative tauopathies: moving toward the specific targeting of the most toxic tau species. ACS Chem. Neurosci. 5, 752–769 (2014).
Soeda, Y. et al. Toxic tau oligomer formation blocked by capping of cysteine residues with 1,2-dihydroxybenzene groups. Nat. Commun. 6, 10216 (2015).
Song, L. et al. Analysis of tau post-translational modifications in rTg4510 mice, a model of tau pathology. Mol. Neurodegener. 10, 14 (2015).
Rocher, A. B. et al. Structural and functional changes in tau mutant mice neurons are not linked to the presence of NFTs. Exp. Neurol. 223, 385–393 (2010).
Santacruz, K. et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005). An inducible mouse model of tauopathy demonstrating that NFTs are not sufficient to cause cognitive decline or neuronal death in tauopathy.
Kuchibhotla, K. V. et al. Neurofibrillary tangle-bearing neurons are functionally integrated in cortical circuits in vivo . Proc. Natl Acad. Sci. USA 111, 510–514 (2014).
Selkoe, D. J. & Hardy, J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 8, 595–608 (2016).
Mairet-Coello, G. et al. The CAMKK2–AMPK kinase pathway mediates the synaptotoxic effects of Aβ oligomers through tau phosphorylation. Neuron 78, 94–108 (2013).
Roberson, E. D. et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science 316, 750–754 (2007). This paper established in vivo that tau reduction protects against amyloid toxicity and excitotoxicity.
Ittner, L. M. & Götz, J. Amyloid-β and tau — a toxic pas de deux in Alzheimer's disease. Nat. Rev. Neurosci. 12, 65–72 (2011).
Warmus, B. A. et al. Tau-mediated NMDA receptor impairment underlies dysfunction of a selectively vulnerable network in a mouse model of frontotemporal dementia. J. Neurosci. 34, 16482–16495 (2014).
Guo, J. L. et al. The dynamics and turnover of tau aggregates in cultured cells: insights into therapies for tauopathies. J. Biol. Chem. 291, 13175–13193 (2016).
Pavlova, A. et al. Protein structural and surface water rearrangement constitute major events in the earliest aggregation stages of tau. Proc. Natl Acad. Sci. USA 113, E127–E136 (2016).
Usenovic, M. et al. Internalized tau oligomers cause neurodegeneration by inducing accumulation of pathogenic tau in human neurons derived from induced pluripotent stem cells. J. Neurosci. 35, 14234–14250 (2015).
Jo, C. et al. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat. Commun. 5, 3496 (2014). This study discloses a role for autophagy-mediated tau degradation in AD.
Stack, C. et al. Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity. Hum. Mol. Genet. 23, 3716–3732 (2014).
Hochgrafe, K. et al. Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human tau. Acta Neuropathol. Commun. 3, 25 (2015).
Rhein, V. et al. Amyloid-β and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc. Natl Acad. Sci. USA 106, 20057–20062 (2009).
Duboff, B., Götz, J. & Feany, M. B. Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron 75, 618–632 (2012).
Manczak, M. & Reddy, P. H. Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer's disease. Hum. Mol. Genet. 21, 5131–5146 (2012).
Corsetti, V. et al. NH2-truncated human tau induces deregulated mitophagy in neurons by aberrant recruitment of Parkin and UCHL-1: implications in Alzheimer's disease. Hum. Mol. Genet. 24, 3058–3081 (2015).
Hu, Y. et al. Tau accumulation impairs mitophagy via increasing mitochondrial membrane potential and reducing mitochondrial Parkin. Oncotarget 7, 17356–17368 (2016).
Frost, B., Hemberg, M., Lewis, J. & Feany, M. B. Tau promotes neurodegeneration through global chromatin relaxation. Nat. Neurosci. 17, 357–366 (2014). This study in D. melanogaster establishes that epigenetic changes in AD are linked to tau-induced heterochromatin loss.
Andorfer, C. et al. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J. Neurosci. 25, 5446–5454 (2005).
Hoozemans, J. J. et al. The unfolded protein response affects neuronal cell cycle protein expression: implications for Alzheimer's disease pathogenesis. Exp. Gerontol. 41, 380–386 (2006).
Yamashima, T. Reconsider Alzheimer's disease by the 'calpain-cathepsin hypothesis' — a perspective review. Prog. Neurobiol. 105, 1–23 (2013).
Maphis, N. et al. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain 138, 1738–1755 (2015).
Wes, P. D., Sayed, F. A., Bard, F. & Gan, L. Targeting microglia for the treatment of Alzheimer's disease. Glia 64, 1710–1732 (2016).
Braak, H. & Braak, E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol. Aging 16, 271–284 (1995).
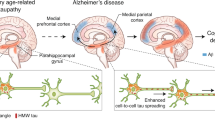
Clavaguera, F., Hench, J., Goedert, M. & Tolnay, M. Invited review: prion-like transmission and spreading of tau pathology. Neuropathol. Appl. Neurobiol. 41, 47–58 (2015).
Medina, M. & Avila, J. The role of extracellular tau in the spreading of neurofibrillary pathology. Front. Cell Neurosci. 8, 113 (2014).
Jucker, M. & Walker, L. C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501, 45–51 (2013).
de Calignon, A. et al. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron 73, 685–697 (2012).
Clavaguera, F. et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl Acad. Sci. USA 110, 9535–9540 (2013). This paper suggests the spreading of tauopathy in a prion-like manner. It further models strain-specific effects of the respective hallmark lesions of different tauopathies.
Ahmed, Z. et al. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 127, 667–683 (2014).
Polanco, J. C., Scicluna, B. J., Hill, A. F. & Götz, J. Extracellular vesicles isolated from the brains of rTg4510 mice seed tau protein aggregation in a threshold-dependent manner. J. Biol. Chem. 291, 12445–12466 (2016).
Wu, J. W. et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci. 19, 1085–1092 (2016).
Wegmann, S. et al. Removing endogenous tau does not prevent tau propagation yet reduces its neurotoxicity. EMBO J. 34, 3028–3041 (2015).
Schirmer, R. H., Adler, H., Pickhardt, M. & Mandelkow, E. “Lest we forget you — methylene blue...”. Neurobiol. Aging 32, 2325.e7–2325.e16 (2011).
Wischik, C. M., Edwards, P. C., Lai, R. Y., Roth, M. & Harrington, C. R. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc. Natl Acad. Sci. USA 93, 11213–11218 (1996). An early in vitro study highlighting the potential of tau aggregation inhibitors in preventing the progression of tauopathy.
van Bebber, F., Paquet, D., Hruscha, A., Schmid, B. & Haass, C. Methylene blue fails to inhibit tau and polyglutamine protein-dependent toxicity in zebrafish. Neurobiol. Dis. 39, 265–271 (2010).
Congdon, E. E. et al. Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy 8, 609–622 (2012).
Fatouros, C. et al. Inhibition of tau aggregation in a novel Caenorhabditis elegans model of tauopathy mitigates proteotoxicity. Hum. Mol. Genet. 21, 3587–3603 (2012).
Baddeley, T. C. et al. Complex disposition of methylthioninium redox forms determines efficacy in tau aggregation inhibitor therapy for Alzheimer's disease. J. Pharmacol. Exp. Ther. 352, 110–118 (2015).
Clifton 2nd, J. & Leikin, J. B. Methylene blue. Am. J. Ther. 10, 289–291 (2003).
Mohideen, S. S., Yamasaki, Y., Omata, Y., Tsuda, L. & Yoshiike, Y. Nontoxic singlet oxygen generator as a therapeutic candidate for treating tauopathies. Sci. Rep. 5, 10821 (2015).
Wagner, J. et al. Reducing tau aggregates with anle138b delays disease progression in a mouse model of tauopathies. Acta Neuropathol. 130, 619–631 (2015).
Wischik, C. M. et al. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer's disease. J. Alzheimers Dis. 44, 705–720 (2015).
Gauthier, S. et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer's disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 388, 2873–2884 (2016).
Morris, M., Maeda, S., Vossel, K. & Mucke, L. The many faces of tau. Neuron 70, 410–426 (2011).
Ma, Q. L. et al. Loss of MAP function leads to hippocampal synapse loss and deficits in the Morris Water Maze with aging. J. Neurosci. 34, 7124–7136 (2014).
van Hummel, A. et al. No overt deficits in aged tau-deficient C57Bl/6. Mapttm1(EGFP)Kit GFP knockin mice. PLoS ONE 11, e0163236 (2016).
Gheyara, A. L. et al. Tau reduction prevents disease in a mouse model of Dravet syndrome. Ann. Neurol. 76, 443–456 (2014).
Xu, H. et al. Tau silencing by siRNA in the P301S mouse model of tauopathy. Curr. Gene Ther. 14, 343–351 (2014).
DeVos, S. L. et al. Antisense reduction of tau in adult mice protects against seizures. J. Neurosci. 33, 12887–12897 (2013).
Smith, P. Y. et al. miR-132/212 deficiency impairs tau metabolism and promotes pathological aggregation in vivo. Hum. Mol. Genet. 24, 6721–6735 (2015).
Lasagna-Reeves, C. A. et al. Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron 92, 407–418 (2016).
Oddo, S., Billings, L., Kesslak, J. P., Cribbs, D. H. & LaFerla, F. M. Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 43, 321–332 (2004).
Pedersen, J. T. & Sigurdsson, E. M. Tau immunotherapy for Alzheimer's disease. Trends Mol. Med. 21, 394–402 (2015).
Rosenmann, H. et al. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch. Neurol. 63, 1459–1467 (2006).
Asuni, A. A., Boutajangout, A., Quartermain, D. & Sigurdsson, E. M. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J. Neurosci. 27, 9115–9129 (2007). A study establishing an immunization that targets a pathological tau epitope to ameliorate tauopathy and cognitive decline in mice.
Boimel, M. et al. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp. Neurol. 224, 472–485 (2010).
Boutajangout, A., Quartermain, D. & Sigurdsson, E. M. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J. Neurosci. 30, 16559–16566 (2010).
Bi, M., Ittner, A., Ke, Y. D., Götz, J. & Ittner, L. M. Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PLoS ONE 6, e26860 (2011).
Troquier, L. et al. Targeting phospho-Ser422 by active tau immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr. Alzheimer Res. 9, 397–405 (2012).
Rozenstein-Tsalkovich, L. et al. Repeated immunization of mice with phosphorylated-tau peptides causes neuroinflammation. Exp. Neurol. 248, 451–456 (2013).
Novak, P. et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer's disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 16, 123–134 (2016).
Boutajangout, A., Ingadottir, J., Davies, P. & Sigurdsson, E. M. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J. Neurochem. 118, 658–667 (2011).
Chai, X. et al. Passive immunization with anti-tau antibodies in two transgenic models: Reduction of tau pathology and delay of disease progression. J. Biol. Chem. 286, 34457–34467 (2011).
Gu, J., Congdon, E. E. & Sigurdsson, E. M. Two novel tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce tau protein pathology. J. Biol. Chem. 288, 33081–33095 (2013).
Collin, L. et al. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer's disease. Brain 137, 2834–2846 (2014).
Kondo, A. et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 523, 431–436 (2015). A proof-of-principle study targeting cis phospho-tau and identifying an antibody for therapeutic intervention.
Congdon, E. E., Gu, J., Sait, H. B. & Sigurdsson, E. M. Antibody uptake into neurons occurs primarily via clathrin-dependent Fcγ receptor endocytosis and is a prerequisite for acute tau protein clearance. J. Biol. Chem. 288, 35452–35465 (2013).
Yanamandra, K. et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 80, 402–414 (2013). Immunotherapy strategy specifically targeting the seeding activity of tau aggregates shows efficacy in blocking the progression of tauopathy.
Funk, K. E., Mirbaha, H., Jiang, H., Holtzman, D. M. & Diamond, M. I. Distinct therapeutic mechanisms of tau antibodies: promoting microglial clearance versus blocking neuronal uptake. J. Biol. Chem. 290, 21652–21662 (2015).
Congdon, E. E. et al. Affinity of tau antibodies for solubilized pathological tau species but not their immunogen or insoluble tau aggregates predicts in vivo and ex vivo efficacy. Mol. Neurodegener. 11, 62 (2016).
Zhang, B. et al. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc. Natl Acad. Sci. USA 102, 227–231 (2005).
Ising, C. et al. AAV-mediated expression of anti-tau scFvs decreases tau accumulation in a mouse model of tauopathy. J. Exp. Med. 214, 1227–1238 (2017).
Nisbet, R. M. et al. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain 140, 1220–1230 (2017).
Hromadkova, L. et al. Identification and characterization of natural antibodies against tau protein in an intravenous immunoglobulin product. J. Neuroimmunol. 289, 121–129 (2015).
Counts, S. E., Perez, S. E., He, B. & Mufson, E. J. Intravenous immunoglobulin reduces tau pathology and preserves neuroplastic gene expression in the 3xTg mouse model of Alzheimer's disease. Curr. Alzheimer Res. 11, 655–663 (2014).
Esteves-Villanueva, J. O., Trzeciakiewicz, H., Loeffler, D. A. & Martic, S. Effects of tau domain-specific antibodies and intravenous immunoglobulin on tau aggregation and aggregate degradation. Biochemistry 54, 293–302 (2015).
Le Corre, S. et al. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc. Natl Acad. Sci. USA 103, 9673–9678 (2006).
Tran, H. T., Sanchez, L. & Brody, D. L. Inhibition of JNK by a peptide inhibitor reduces traumatic brain injury-induced tauopathy in transgenic mice. J. Neuropathol. Exp. Neurol. 71, 116–129 (2012).
Ittner, A. et al. Site-specific phosphorylation of tau inhibits amyloid-beta toxicity in Alzheimer's mice. Science 354, 904–908 (2016). This study challenges the oversimplification of a generally disease-promoting effect of tau phosphorylation.
Zhang, X. et al. Diaminothiazoles modify tau phosphorylation and improve the tauopathy in mouse models. J. Biol. Chem. 288, 22042–22056 (2013).
Li, L. et al. Ginsenoside Rd attenuates β-amyloid-induced tau phosphorylation by altering the functional balance of glycogen synthase kinase 3β and protein phosphatase 2A. Neurobiol. Dis. 54, 320–328 (2013).
Cisse, M. et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature 469, 47–52 (2011). This paper identified a loss of function of EphB2 in mediating AD-related neuronal dysfunction.
Jiang, J. et al. Stimulation of EphB2 attenuates tau phosphorylation through PI3K/Akt-mediated inactivation of glycogen synthase kinase-3β. Sci. Rep. 5, 11765 (2015).
Myeku, N. et al. Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP–PKA signaling. Nat. Med. 22, 46–53 (2016).
Martinez, A., Gil, C. & Perez, D. I. Glycogen synthase kinase 3 inhibitors in the next horizon for Alzheimer's disease treatment. Int. J. Alzheimers Dis. 2011, 280502 (2011).
Dominguez, J. M. et al. Evidence for irreversible inhibition of glycogen synthase kinase-3β by tideglusib. J. Biol. Chem. 287, 893–904 (2012).
Lovestone, S. et al. A phase II trial of tideglusib in Alzheimer's disease. J. Alzheimers Dis. 45, 75–88 (2015).
Tolosa, E. et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord. 29, 470–478 (2014).
Mullard, A. Pharma pumps up anti-tau Alzheimer pipeline despite first phase III failure. Nat. Rev. Drug Discov. 15, 591–592 (2016).
Lahmy, V. et al. Blockade of tau hyperphosphorylation and Aβ1–42 generation by the aminotetrahydrofuran derivative ANAVEX2-73, a mixed muscarinic and σ1 receptor agonist, in a nontransgenic mouse model of Alzheimer's disease. Neuropsychopharmacology 38, 1706–1723 (2013).
Kaufman, A. C. et al. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Ann. Neurol. 77, 953–971 (2015).
Gentry, E. G. et al. Rho kinase inhibition as a therapeutic for progressive supranuclear palsy and corticobasal degeneration. J. Neurosci. 36, 1316–1323 (2016).
Katz, J. D. et al. Structure guided design of a series of selective pyrrolopyrimidinone MARK inhibitors. Bioorg. Med. Chem. Lett. 27, 114–120 (2017).
Vogelsberg-Ragaglia, V., Schuck, T., Trojanowski, J. Q. & Lee, V. M. PP2A mRNA expression is quantitatively decreased in Alzheimer's disease hippocampus. Exp. Neurol. 168, 402–412 (2001).
van Eersel, J. et al. Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer's disease models. Proc. Natl Acad. Sci. USA 107, 13888–13893 (2010).
Liu, S. J. et al. Sodium selenate retards epileptogenesis in acquired epilepsy models reversing changes in protein phosphatase 2A and hyperphosphorylated tau. Brain 139, 1919–1938 (2016).
Shultz, S. R. et al. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain 138, 1297–1313 (2015).
Lu, P. J., Wulf, G., Zhou, X. Z., Davies, P. & Lu, K. P. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 399, 784–788 (1999).
Min, S. W. et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953–966 (2010).
Tracy, T. E. et al. Acetylated Tau obstructs KIBRA-mediated signaling in synaptic plasticity and promotes tauopathy-related memory loss. Neuron 90, 245–260 (2016). A study describing the disruption of synaptic transmission by acetylated tau.
Xiong, Y. et al. HDAC6 mutations rescue human tau-induced microtubule defects in Drosophila. Proc. Natl Acad. Sci. USA 110, 4604–4609 (2013).
Cole, G. M. et al. Prevention of Alzheimer's disease: omega-3 fatty acid and phenolic anti-oxidant interventions. Neurobiol. Aging 26 (Suppl. 1), 133–136 (2005).
Miyasaka, T. et al. Curcumin improves tau-induced neuronal dysfunction of nematodes. Neurobiol. Aging 39, 69–81 (2016).
Giannopoulos, P. F. et al. Pharmacologic inhibition of 5-lipoxygenase improves memory, rescues synaptic dysfunction, and ameliorates tau pathology in a transgenic model of tauopathy. Biol. Psychiatry 78, 693–701 (2015).
Chu, J. et al. Pharmacologic blockade of 12/15-lipoxygenase ameliorates memory deficits, Aβ and tau neuropathology in the triple-transgenic mice. Mol. Psychiatry 20, 1329–1338 (2015).
Ringman, J. M. et al. Oral curcumin for Alzheimer's disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 4, 43 (2012).
Galasko, D. R. et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch. Neurol. 69, 836–841 (2012).
Schaeffer, V. et al. Stimulation of autophagy reduces neurodegeneration in a mouse model of human tauopathy. Brain 135, 2169–2177 (2012).
Ozcelik, S. et al. Rapamycin attenuates the progression of tau pathology in P301S tau transgenic mice. PLoS ONE 8, e62459 (2013).
Frederick, C. et al. Rapamycin ester analog CCI-779/Temsirolimus alleviates tau pathology and improves motor deficit in mutant tau transgenic mice. J. Alzheimers Dis. 44, 1145–1156 (2015).
Noack, M. & Richter-Landsberg, C. Activation of autophagy by rapamycin does not protect oligodendrocytes against protein aggregate formation and cell death induced by proteasomal inhibition. J. Mol. Neurosci. 55, 99–108 (2015).
Kim, S. et al. Fisetin stimulates autophagic degradation of phosphorylated tau via the activation of TFEB and Nrf2 transcription factors. Sci. Rep. 6, 24933 (2016).
Lei, Z., Brizzee, C. & Johnson, G. V. BAG3 facilitates the clearance of endogenous tau in primary neurons. Neurobiol. Aging 36, 241–248 (2015).
Drubin, D. G. & Kirschner, M. W. Tau protein function in living cells. J. Cell Biol. 103, 2739–2746 (1986).
Lovestone, S., Hartley, C. L., Pearce, J. & Anderton, B. H. Phosphorylation of tau by glycogen synthase kinase-3β in intact mammalian cells: the effects on the organization and stability of microtubules. Neuroscience 73, 1145–1157 (1996).
Brunden, K. R. et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J. Neurosci. 30, 13861–13866 (2010).
Gozes, I. Microtubules (tau) as an emerging therapeutic target: NAP (davunetide). Curr. Pharm. Des. 17, 3413–3417 (2011).
Quraishe, S., Cowan, C. M. & Mudher, A. NAP (davunetide) rescues neuronal dysfunction in a Drosophila model of tauopathy. Mol. Psychiatry 18, 834–842 (2013).
Morimoto, B. H. et al. A double-blind, placebo-controlled, ascending-dose, randomized study to evaluate the safety, tolerability and effects on cognition of AL-108 after 12 weeks of intranasal administration in subjects with mild cognitive impairment. Dement Geriatr. Cogn. Disord. 35, 325–336 (2013).
Boxer, A. L. et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 13, 676–685 (2014).
Rao, M. V. et al. Specific calpain inhibition by calpastatin prevents tauopathy and neurodegeneration and restores normal lifespan in tau P301L mice. J. Neurosci. 34, 9222–9234 (2014).
Medeiros, R. et al. Calpain inhibitor A-705253 mitigates Alzheimer's disease-like pathology and cognitive decline in aged 3xTgAD mice. Am. J. Pathol. 181, 616–625 (2012).
Rockenstein, E. et al. Neuroprotective effects of cerebrolysin in triple repeat Tau transgenic model of Pick's disease and fronto-temporal tauopathies. BMC Neurosci. 16, 85 (2015).
Wu, Y. et al. Intraperitoneal administration of a novel TAT-BDNF peptide ameliorates cognitive impairments via modulating multiple pathways in two Alzheimer's rodent models. Sci. Rep. 5, 15032 (2015).
Banzhaf-Strathmann, J. et al. MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer's disease. EMBO J. 33, 1667–1680 (2014). This study demonstrated that an AD-associated miRNA contributes to tauopathy and memory impairment.
Santa-Maria, I. et al. Dysregulation of microRNA-219 promotes neurodegeneration through post-transcriptional regulation of tau. J. Clin. Invest. 125, 681–686 (2015).
Zumkehr, J. et al. Ceftriaxone ameliorates tau pathology and cognitive decline via restoration of glial glutamate transporter in a mouse model of Alzheimer's disease. Neurobiol. Aging 36, 2260–2271 (2015).
Barini, E. et al. Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol. Neurodegener. 11, 16 (2016).
Ke, Y. D., Delerue, F., Gladbach, A., Götz, J. & Ittner, L. M. Experimental diabetes mellitus exacerbates tau pathology in a transgenic mouse model of Alzheimer's disease. PLoS ONE 4, e7917 (2009).
Leinenga, G., Langton, C., Nisbet, R. & Götz, J. Ultrasound treatment of neurological diseases - current and emerging applications. Nat. Rev. Neurol. 12, 161–174 (2016).
Yu, Y. J. et al. Therapeutic bispecific antibodies cross the blood–brain barrier in nonhuman primates. Sci. Transl Med. 6, 261ra154 (2014).
Leinenga, G. & Götz, J. Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer's disease mouse model. Sci. Transl Med. 7, 278ra233 (2015).
Montagne, A. et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Halliday, M. R. et al. Accelerated pericyte degeneration and blood–brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J. Cereb. Blood Flow Metab. 36, 216–227 (2016).
Bien-Ly, N. et al. Lack of widespread BBB disruption in Alzheimer's disease models: focus on therapeutic antibodies. Neuron 88, 289–297 (2015).
Yamin, G. & Teplow, D. B. Pittsburgh Compound-B (PiB) binds amyloid β-protein protofibrils. J. Neurochem. 140, 210–215 (2016).
Mattsson, N. et al. Revolutionizing Alzheimer's disease and clinical trials through biomarkers. Alzheimers Dement. 1, 412–419 (2015).
Harada, R. et al. Characteristics of tau and its ligands in PET imaging. Biomolecules 6, 7 (2016).
Scholl, M. et al. PET imaging of tau deposition in the aging human brain. Neuron 89, 971–982 (2016).
Schwarz, A. J. et al. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain 139, 1539–1550 (2016).
Xia, C. et al. Association of in vivo18FAV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol. 74, 427–436 (2017).
Ossenkoppele, R. et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain 139, 1551–1567 (2016).
Lleo, A. et al. Cerebrospinal fluid biomarkers in trials for Alzheimer and Parkinson diseases. Nat. Rev. Neurol. 11, 41–55 (2015).
Vos, S. J. et al. Prediction of Alzheimer disease in subjects with amnestic and nonamnestic MCI. Neurology 80, 1124–1132 (2013).
Kuiperij, H. B. et al. Tau rather than TDP-43 proteins are potential cerebrospinal fluid biomarkers for frontotemporal lobar degeneration subtypes: a pilot study. J. Alzheimers Dis. 55, 585–595 (2017).
Pandey, S. et al. A prospective pilot study on serum cleaved tau protein as a neurological marker in severe traumatic brain injury. Br. J. Neurosurg. 1, 1–8 (2017).
Wang, S. X. et al. Detection of the tau protein in human serum by a sensitive four-electrode electrochemical biosensor. Biosens. Bioelectron. 92, 482–488 (2017).
Yanamandra, K. et al. Anti-tau antibody administration increases plasma tau in transgenic mice and patients with tauopathy. Sci. Transl Med. 9, eaal2029 (2017).
Hampel, H. et al. Precision medicine — the golden gate for detection, treatment and prevention of Alzheimer's disease. J. Prev. Alzheimers Dis. 3, 243–259 (2016).
Lawler, M. & Sullivan, R. Personalised and precision medicine in cancer clinical trials: panacea for progress or Pandora's Box? Public Health Genomics 18, 329–337 (2015).
Bateman, R. J. et al. The DIAN-TU Next Generation Alzheimer's prevention trial: adaptive design and disease progression model. Alzheimers Dement. 13, 8–19 (2016).
Hendrix, J. A. et al. Challenges, solutions, and recommendations for Alzheimer's disease combination therapy. Alzheimers Dement. 12, 623–630 (2016).
Götz, J. & Ittner, L. M. Animal models of Alzheimer's disease and frontotemporal dementia. Nat. Rev. Neurosci. 9, 532–544 (2008).
Jadhav, S. et al. Truncated tau deregulates synaptic markers in rat model for human tauopathy. Front. Cell Neurosci. 9, 24 (2015).
van Groen, T. et al. Age-related brain pathology in Octodon degu: blood vessel, white matter and Alzheimer-like pathology. Neurobiol. Aging 32, 1651–1661 (2011).
Orr, M. E., Garbarino, V. R., Salinas, A. & Buffenstein, R. Sustained high levels of neuroprotective, high molecular weight, phosphorylated tau in the longest-lived rodent. Neurobiol. Aging 36, 1496–1504 (2015).
Jackson, S. J. et al. Does age matter? The impact of rodent age on study outcomes. Lab. Anim. 51, 160–169 (2016).
Xia, D., Gutmann, J. M. & Götz, J. Mobility and subcellular localization of endogenous, gene-edited tau differs from that of over-expressed human wild-type and P301L mutant tau. Sci. Rep. 6, 29074 (2016).
Saito, T., Matsuba, Y., Yamazaki, N., Hashimoto, S. & Saido, T. C. Calpain activation in Alzheimer's model mice is an artifact of APP and presenilin overexpression. J. Neurosci. 36, 9933–9936 (2016).
Scudellari, M. How iPS cells changed the world. Nature 534, 310–312 (2016).
Paquet, D. et al. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature 533, 125–129 (2016). The gene-editing tool CRISPR–Cas9 was applied to human iPS cells by introducing AD-causing mutations with high efficiency to recapitulate diseased-associated phenotypes.
Choi, S. H. et al. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature 515, 274–278 (2014).
Furman, J. L. et al. Widespread tau seeding activity at early Braak stages. Acta Neuropathol. 133, 91–100 (2016).
Holmes, B. B. et al. Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl Acad. Sci. USA 111, E4376–E4385 (2014).
Ahn, M. et al. Brain aggregates: an effective in vitro cell culture system modeling neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 75, 256–262 (2016).
Solomon, B., Koppel, R., Frankel, D. & Hanan-Aharon, E. Disaggregation of Alzheimer β-amyloid by site-directed mAb. Proc. Natl Acad. Sci. USA 94, 4109–4112 (1997).
Schenk, D. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 (1999).
Bard, F. et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 6, 916–919 (2000).
Pan, W., Solomon, B., Maness, L. M. & Kastin, A. J. Antibodies to β-amyloid decrease the blood-to-brain transfer of β-amyloid peptide. Exp. Biol. Med. 227, 609–615 (2002).
Golde, T. E. Open questions for Alzheimer's disease immunotherapy. Alzheimers Res. Ther. 6, 3 (2014).
Orgogozo, J. M. et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology 61, 46–54 (2003).
Hock, C. et al. Antibodies against β-amyloid slow cognitive decline in Alzheimer's disease. Neuron 38, 547–554 (2003).
Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature 537, 50–56 (2016).
Hanenberg, M. et al. Amyloid-β peptide-specific DARPins as a novel class of potential therapeutics for Alzheimer disease. J. Biol. Chem. 289, 27080–27089 (2014).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Mullard, A. Stem-cell discovery platforms yield first clinical candidates. Nat. Rev. Drug Discov. 14, 589–591 (2015).
Acknowledgements
The authors acknowledge support by the Estate of Dr Clem Jones AO, the Government of Queensland (DSITI), the Australian Research Council (DP160103812) and the National Health and Medical Research Council of Australia (GNT1037746, GNT1127999). The authors thank R. Tweedale for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Alternative splicing
-
A mechanism by which exons or portions of exons or non-coding regions within a pre-mRNA that is transcribed from a single gene are differentially joined or skipped, resulting in transcripts from which multiple protein isoforms are generated.
- Haplotypes
-
Groups of alleles of different genes on a single chromosome that are linked closely enough to be inherited together; for example, the linked genes of the major histocompatibility complex.
- Interactome
-
All the interactions between biological entities in cells and organisms considered as a whole.
- Overexpression artefact
-
A phenotype seen in transgenic animal models that potentially results from the expression of the transgene at higher levels than the endogenous protein, owing to the choice of promoter for transgene expression, the chromosomal integration site of the transgene and the copy number.
- Long-term depression
-
A cellular mechanism underlying learning and memory that involves an activity-dependent reduction in synaptic efficacy that lasts hours or longer following a long patterned stimulus.
- Long-term potentiation
-
A cellular mechanism underlying learning and memory that involves a persistent increase in synaptic strength following high-frequency stimulation.
- Braak staging
-
A system formulated by Braak and Braak for staging Alzheimer disease severity using a tau-specific antibody on brain sections, based on the premise that tau pathology spreads sequentially from the mesial temporal lobe (stages I and II), extending to the limbic regions (stages III and IV, when dementia manifests) and then the neocortex (stages V and VI).
- Cis/trans-isomerase
-
A type of enzyme that catalyses the isomerization of geometric isomers, thereby affecting the activity of conformation-specific enzymes such as protein phosphatase 2A (PP2A).
Rights and permissions
About this article
Cite this article
Li, C., Götz, J. Tau-based therapies in neurodegeneration: opportunities and challenges. Nat Rev Drug Discov 16, 863–883 (2017). https://doi.org/10.1038/nrd.2017.155
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2017.155
This article is cited by
-
TFR1 knockdown alleviates iron overload and mitochondrial dysfunction during neural differentiation of Alzheimer’s disease-derived induced pluripotent stem cells by interacting with GSK3B
European Journal of Medical Research (2024)
-
Tau reduction attenuates autism-like features in Fmr1 knockout mice
Molecular Autism (2023)
-
Intermittent hypoxia therapy ameliorates beta-amyloid pathology via TFEB-mediated autophagy in murine Alzheimer's disease
Journal of Neuroinflammation (2023)
-
The therapeutic landscape of tauopathies: challenges and prospects
Alzheimer's Research & Therapy (2023)
-
Tau and neuroinflammation in Alzheimer’s disease: interplay mechanisms and clinical translation
Journal of Neuroinflammation (2023)