Key Points
-
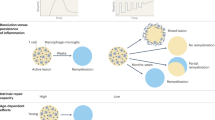
People with multiple sclerosis exhibit variable degrees of remyelination.
-
Individuals with greater capacity to remyelinate seem to have less clinical disability, thereby fuelling the research to find remyelination therapeutics for multiple sclerosis.
-
The biology of remyelination and its similarities and differences from developmental myelination are discussed.
-
Many pro-remyelinating therapeutics have been identified from experimental models.
-
Early phase clinical trials with potential pro-remyelinating therapeutics are in progress.
-
Many challenges remain on how best to perform clinical trials of potential pro-remyelinating medications for multiple sclerosis.
Abstract
Multiple sclerosis is characterized by inflammatory activity that results in destruction of the myelin sheaths that enwrap axons. The currently available medications for multiple sclerosis are predominantly immune-modulating and do not directly promote repair. White matter regeneration, or remyelination, is a new and exciting potential approach to treating multiple sclerosis, as remyelination repairs the damaged regions of the central nervous system. A wealth of new strategies in animal models that promote remyelination, including the repopulation of oligodendrocytes that produce myelin, has led to several clinical trials to test new reparative therapies. In this Review, we highlight the biology of, and obstacles to, remyelination. We address new strategies to improve remyelination in preclinical models, highlight the therapies that are currently undergoing clinical trials and discuss the challenges of objectively measuring remyelination in trials of repair in multiple sclerosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bray, G. M., Rasminsky, M. & Aguayo, A. J. Interactions between axons and their sheath cells. Annu. Rev. Neurosci. 4, 127–162 (1981).
Bunge, R. P. Glial cells and the central myelin sheath. Physiol. Rev. 48, 197–251 (1968).
Nave, K. A. & Trapp, B. D. Axon-glial signaling and the glial support of axon function. Annu. Rev. Neurosci. 31, 535–561 (2008).
Nave, K. A. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 11, 275–283 (2010).
Griffiths, I. et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 280, 1610–1613 (1998).
Lappe-Siefke, C. et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 33, 366–374 (2003).
Nguyen, T. et al. Axonal protective effects of the myelin-associated glycoprotein. J. Neurosci. 29, 630–637 (2009).
Locatelli, G. et al. Primary oligodendrocyte death does not elicit anti-CNS immunity. Nat. Neurosci. 15, 543–550 (2012).
Pohl, H. B. et al. Genetically induced adult oligodendrocyte cell death is associated with poor myelin clearance, reduced remyelination, and axonal damage. J. Neurosci. 31, 1069–1080 (2011).
Ghosh, A. et al. Targeted ablation of oligodendrocytes triggers axonal damage. PLoS ONE 6, e22735 (2011).
Oluich, L. J. et al. Targeted ablation of oligodendrocytes induces axonal pathology independent of overt demyelination. J. Neurosci. 32, 8317–8330 (2012).
Smith, C. M., Cooksey, E. & Duncan, I. D. Myelin loss does not lead to axonal degeneration in a long-lived model of chronic demyelination. J. Neurosci. 33, 2718–2727 (2013). This study found no signs of axonal degeneration at 9 months of age in transgenic rats with chronic demyelination, suggesting that axons can remain healthy in the absence of myelin.
Manrique-Hoyos, N. et al. Late motor decline after accomplished remyelination: impact for progressive multiple sclerosis. Ann. Neurol. 71, 227–244 (2012).
Funfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012). The lack of demyelination and white matter injury in transgenic mice in which the oligodendrocytes lack mitochondria demonstrates that oligodendrocytes survive by converting glucose to lactate, even in the presence of oxygen. Lactate is thought to be a nutrient source for the underlying axon.
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012). The most abundant lactate transporter, MCT1, is enriched in oligodendrocytes and reducing oligodendrocyte MCT1 results in axonal injury, thus demonstrating a role for oligodendrocytes in supporting axons.
Micu, I. et al. The molecular physiology of the axo-myelinic synapse. Exp. Neurol. 276, 41–50 (2016). This paper describes a new form of communication between the axon and myelin, whereby axonal activity drives glutamate release along the length of the axon, which activates myelinic glutamatergic receptors.
Saab, A. S. et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132 (2016). Coordination of axonal activity and oligodendrocyte metabolic release was revealed to be dependent on oligodendrocyte NMDA receptor activation. Glutamate activation of NMDA receptor mobilizes glucose transporter GLUT1, which enhanced lactate support to fast-spiking axons.
Fruhbeis, C. et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 11, e1001604 (2013).
Shakhbazau, A. et al. Demyelination induces transport of ribosome-containing vesicles from glia to axons: evidence from animal models and MS patient brains. Mol. Biol. Rep. 43, 495–507 (2016).
Dai, X. et al. The trophic role of oligodendrocytes in the basal forebrain. J. Neurosci. 23, 5846–5853 (2003).
Wilkins, A., Majed, H., Layfield, R., Compston, A. & Chandran, S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J. Neurosci. 23, 4967–4974 (2003).
Wilkins, A., Chandran, S. & Compston, A. A role for oligodendrocyte-derived IGF-1 in trophic support of cortical neurons. Glia 36, 48–57 (2001).
Lu, Q. R. et al. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron 25, 317–329 (2000).
Zhou, Q., Wang, S. & Anderson, D. J. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron 25, 331–343 (2000).
Yue, T. et al. A critical role for dorsal progenitors in cortical myelination. J. Neurosci. 26, 1275–1280 (2006).
Tsai, H. H. et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 351, 379–384 (2016).
Kirby, B. B. et al. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci. 9, 1506–1511 (2006).
de Castro, F., Bribian, A. & Ortega, M. C. Regulation of oligodendrocyte precursor migration during development, in adulthood and in pathology. Cell. Mol. Life Sci. 70, 4355–4368 (2013).
Hughes, E. G., Kang, S. H., Fukaya, M. & Bergles, D. E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 16, 668–676 (2013). This study uses live two-photon imaging of cortical OPCs to demonstrate that OPCs in vivo are highly motile and locally survey their environment but maintain non-overlapping territories through self-avoidance. OPCs rapidly respond to focal injury.
Barnabe-Heider, F. et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7, 470–482 (2010).
Menn, B. et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 26, 7907–7918 (2006).
Psachoulia, K., Jamen, F., Young, K. M. & Richardson, W. D. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 5, 57–67 (2009).
Rivers, L. E. et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11, 1392–1401 (2008).
Jablonska, B. et al. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat. Neurosci. 13, 541–550 (2010).
Xing, Y. L. et al. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J. Neurosci. 34, 14128–14146 (2014).
Ruckh, J. M. et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 (2012). Age is an important contributor to impaired remyelination in MS. This study demonstrates that the age-dependent decrease in remyelination can be rejuvenated by exposure to a youthful systemic milieu.
Sim, F. J., Zhao, C., Penderis, J. & Franklin, R. J. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J. Neurosci. 22, 2451–2459 (2002).
Rosenberg, S. S., Kelland, E. E., Tokar, E., De la Torre, A. R. & Chan, J. R. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc. Natl Acad. Sci. USA 105, 14662–14667 (2008). This study is the first to demonstrate that myelination can occur in fixed axons, suggesting that myelination proceeds in the absence of axonal signals. Instead, myelination is induced by packing restraints imposed on myelinating cells.
Jacobson, S. Sequence of myelinization in the brain of the albino rat. A. Cerebral cortex, thalamus and related structures. J. Comp. Neurol. 121, 5–29 (1963).
Kang, S. H., Fukaya, M., Yang, J. K., Rothstein, J. D. & Bergles, D. E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68, 668–681 (2010).
Young, K. M. et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885 (2013).
Yeung, M. S. et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774 (2014).
Paus, T. et al. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res. Bull. 54, 255–266 (2001).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
Czopka, T., ffrench-Constant, C. & Lyons, D. A. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev. Cell 25, 599–609 (2013). Zebrafish developmental myelination proceeds nearly synchronously for a given oligodendrocyte, with new sheath formation occurring within a window of only a few hours, after which no new sheaths are generated.
Crawford, A. H. et al. Pre-existing mature oligodendrocytes do not contribute to remyelination following toxin-induced spinal cord demyelination. Am. J. Pathol. 186, 511–516 (2016).
Keirstead, H. S. & Blakemore, W. F. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J. Neuropathol. Exp. Neurol. 56, 1191–1201 (1997).
Mensch, S. et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630 (2015).
Hines, J. H., Ravanelli, A. M., Schwindt, R., Scott, E. K. & Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 (2015).
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014). This study uses optogenetic stimulation of cortical neurons to demonstrate that axonal activity increases OPC proliferation and oligodendrogenesis, while also boosting myelination.
Jensen, S. K. & Yong, V. W. Activity-dependent and experience-driven myelination provide new directions for the management of multiple sclerosis. Trends Neurosci. 39, 356–365 (2016).
Koudelka, S. et al. Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr. Biol. 26, 1447–1455 (2016).
Bechler, M. E., Byrne, L. & ffrench-Constant, C. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr. Biol. 25, 2411–2416 (2015).
Lee, S. et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917–922 (2012).
Lundgaard, I. et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743 (2013).
Chong, S. Y. et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc. Natl Acad. Sci. USA 109, 1299–1304 (2012).
Wang, S. et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21, 63–75 (1998).
Zhang, Y. et al. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc. Natl Acad. Sci. USA 106, 19162–19167 (2009).
Hammond, T. R. et al. Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron 81, 588–602 (2014).
Fancy, S. P. et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 23, 1571–1585 (2009).
Fancy, S. P. et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 14, 1009–1016 (2011).
Keough, M. B. et al. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat. Commun. 7, 11312 (2016). By screening medications on OPCs plated on an inhibitory environment containing CSPGs, this study found that some promising oligodendrocyte differentiation compounds were unable to rescue cells from this inhibitory environment. This study also introduced a novel CSPG inhibitor to promote remyelination.
Lau, L. W. et al. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann. Neurol. 72, 419–432 (2012).
Back, S. A. et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 11, 966–972 (2005). This article demonstrates that hyaluronan accumulates in demyelinated MS lesions and impairs remyelination in an animal model.
Sloane, J. A. et al. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc. Natl Acad. Sci. USA 107, 11555–11560 (2010).
Mi, S. et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 8, 745–751 (2005). This paper demonstrates for the first time that LINGO1 is a negative regulator of oligodendrocyte differentiation and myelination.
Mi, S. et al. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann. Neurol. 65, 304–315 (2009).
Plemel, J. R., Manesh, S. B., Sparling, J. S. & Tetzlaff, W. Myelin inhibits oligodendroglial maturation and regulates oligodendrocytic transcription factor expression. Glia 61, 1471–1487 (2013).
Baer, A. S. et al. Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain 132, 465–481 (2009).
McDonald, W. I. & Sears, T. A. Effect of demyelination on conduction in the central nervous system. Nature 221, 182–183 (1969).
Rasminsky, M. & Sears, T. A. Internodal conduction in undissected demyelinated nerve fibres. J. Physiol. 227, 323–350 (1972).
Waxman, S. G. Conduction in myelinated, unmyelinated, and demyelinated fibers. Arch. Neurol. 34, 585–589 (1977).
Jenkins, T. et al. Dissecting structure-function interactions in acute optic neuritis to investigate neuroplasticity. Hum. Brain Mapp. 31, 276–286 (2010).
Smith, K. J. & McDonald, W. I. The pathophysiology of multiple sclerosis: the mechanisms underlying the production of symptoms and the natural history of the disease. Phil. Trans. R. Soc. Lond. B 354, 1649–1673 (1999).
Ritchie, J. M. & Rogart, R. B. Density of sodium channels in mammalian myelinated nerve fibers and nature of the axonal membrane under the myelin sheath. Proc. Natl Acad. Sci. USA 74, 211–215 (1977).
Wang, H., Allen, M. L., Grigg, J. J., Noebels, J. L. & Tempel, B. L. Hypomyelination alters K+ channel expression in mouse mutants shiverer and Trembler. Neuron 15, 1337–1347 (1995).
Wang, H., Kunkel, D. D., Martin, T. M., Schwartzkroin, P. A. & Tempel, B. L. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature 365, 75–79 (1993).
Bostock, H., Sherratt, R. M. & Sears, T. A. Overcoming conduction failure in demyelinated nerve fibres by prolonging action potentials. Nature 274, 385–387 (1978).
Schauf, C. L. & Davis, F. A. Impulse conduction in multiple sclerosis: a theoretical basis for modification by temperature and pharmacological agents. J. Neurol. Neurosurg. Psychiatry 37, 152–161 (1974).
Bostock, H., Sears, T. A. & Sherratt, R. M. The effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. J. Physiol. 313, 301–315 (1981).
Sherratt, R. M., Bostock, H. & Sears, T. A. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature 283, 570–572 (1980).
Duncan, I. D., Brower, A., Kondo, Y., Curlee, J. F. Jr & Schultz, R. D. Extensive remyelination of the CNS leads to functional recovery. Proc. Natl Acad. Sci. USA 106, 6832–6836 (2009).
Traka, M. et al. A genetic mouse model of adult-onset, pervasive central nervous system demyelination with robust remyelination. Brain 133, 3017–3029 (2010).
Mozafari, S., Sherafat, M. A., Javan, M., Mirnajafi-Zadeh, J. & Tiraihi, T. Visual evoked potentials and MBP gene expression imply endogenous myelin repair in adult rat optic nerve and chiasm following local lysolecithin induced demyelination. Brain Res. 1351, 50–56 (2010).
Redford, E. J., Kapoor, R. & Smith, K. J. Nitric oxide donors reversibly block axonal conduction: demyelinated axons are especially susceptible. Brain 120, 2149–2157 (1997).
Hunanyan, A. S. et al. Role of chondroitin sulfate proteoglycans in axonal conduction in mammalian spinal cord. J. Neurosci. 30, 7761–7769 (2010).
Petrosyan, H. A. et al. Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats. J. Neurosci. 33, 4032–4043 (2013).
Felts, P. A., Baker, T. A. & Smith, K. J. Conduction in segmentally demyelinated mammalian central axons. J. Neurosci. 17, 7267–7277 (1997).
Foster, R. E., Whalen, C. C. & Waxman, S. G. Reorganization of the axon membrane in demyelinated peripheral nerve fibers: morphological evidence. Science 210, 661–663 (1980).
England, J. D., Gamboni, F. & Levinson, S. R. Increased numbers of sodium channels form along demyelinated axons. Brain Res. 548, 334–337 (1991).
Black, J. A., Newcombe, J., Trapp, B. D. & Waxman, S. G. Sodium channel expression within chronic multiple sclerosis plaques. J. Neuropathol. Exp. Neurol. 66, 828–837 (2007).
Irvine, K. A. & Blakemore, W. F. Remyelination protects axons from demyelination-associated axon degeneration. Brain 131, 1464–1477 (2008).
Stys, P. K., Waxman, S. G. & Ransom, B. R. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na+-Ca2+ exchanger. J. Neurosci. 12, 430–439 (1992).
Stys, P. K., Ransom, B. R. & Waxman, S. G. Tertiary and quaternary local anesthetics protect CNS white matter from anoxic injury at concentrations that do not block excitability. J. Neurophysiol. 67, 236–240 (1992).
Fern, R., Ransom, B. R., Stys, P. K. & Waxman, S. G. Pharmacological protection of CNS white matter during anoxia: actions of phenytoin, carbamazepine and diazepam. J. Pharmacol. Exp. Ther. 266, 1549–1555 (1993).
Black, J. A., Liu, S., Carrithers, M., Carrithers, L. M. & Waxman, S. G. Exacerbation of experimental autoimmune encephalomyelitis after withdrawal of phenytoin and carbamazepine. Ann. Neurol. 62, 21–33 (2007).
Morsali, D. et al. Safinamide and flecainide protect axons and reduce microglial activation in models of multiple sclerosis. Brain 136, 1067–1082 (2013).
Bechtold, D. A., Kapoor, R. & Smith, K. J. Axonal protection using flecainide in experimental autoimmune encephalomyelitis. Ann. Neurol. 55, 607–616 (2004).
Black, J. A., Liu, S., Hains, B. C., Saab, C. Y. & Waxman, S. G. Long-term protection of central axons with phenytoin in monophasic and chronic-relapsing EAE. Brain 129, 3196–3208 (2006).
Mei, F. et al. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. eLife 5, e18246 (2016).
Patrikios, P. et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain 129, 3165–3172 (2006). By examining the distribution of remyelination in 51 autopsies of patients, this study demonstrates the highly variable nature of remyelination in patients with MS.
Bramow, S. et al. Demyelination versus remyelination in progressive multiple sclerosis. Brain 133, 2983–2998 (2010). This paper demonstrated that lower spinal cord remyelination is associated with a higher level of disability and that the remyelinated plaque is more vulnerable to demyelination than is normal-appearing white matter.
Prineas, J. W., Barnard, R. O., Kwon, E. E., Sharer, L. R. & Cho, E. S. Multiple sclerosis: remyelination of nascent lesions. Ann. Neurol. 33, 137–151 (1993).
Patani, R., Balaratnam, M., Vora, A. & Reynolds, R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol. Appl. Neurobiol. 33, 277–287 (2007).
Blakemore, W. F. Pattern of remyelination in the CNS. Nature 249, 577–578 (1974).
Barkhof, F. et al. Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance. Arch. Neurol. 60, 1073–1081 (2003).
Bodini, B. et al. Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann. Neurol. 79, 726–738 (2016).
Kornek, B. et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 157, 267–276 (2000).
Kidd, D. et al. Cortical lesions in multiple sclerosis. Brain 122, 17–26 (1999).
Kutzelnigg, A. et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128, 2705–2712 (2005).
Vercellino, M. et al. Grey matter pathology in multiple sclerosis. J. Neuropathol. Exp. Neurol. 64, 1101–1107 (2005).
Bai, C. B. et al. A mouse model for testing remyelinating therapies. Exp. Neurol. 283, 330–340 (2016).
Bø, L., Vedeler, C. A., Nyland, H. I., Trapp, B. D. & Mørk, S. J. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J. Neuropathol. Exp. Neurol. 62, 723–732 (2003).
Gilmore, C. P. et al. Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: a comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J. Neurol. Neurosurg. Psychiatry 80, 182–187 (2009).
Roosendaal, S. D. et al. Accumulation of cortical lesions in MS: relation with cognitive impairment. Mult. Scler. 15, 708–714 (2009).
Kilsdonk, I. D. et al. Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: a post-mortem verification study. Brain 139, 1472–1481 (2016).
Chang, A. et al. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann. Neurol. 72, 918–926 (2012). Observations from lesions that span both white and grey matter suggest that grey matter remyelinates more rapidly than white matter, potentially in part because of a lower level of lesional inhibitors.
Goldschmidt, T., Antel, J., Konig, F. B., Bruck, W. & Kuhlmann, T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology 72, 1914–1921 (2009).
Frischer, J. M. et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 78, 710–721 (2015). This article characterized the attributes of ~2,500 MS lesions in relation to clinical data and found that age, gender, disease duration and clinical course all contribute to the evolving white matter pathology in MS.
Brown, R. A., Narayanan, S., Banwell, B., Arnold, D. L. & Canadian Pediatric Demyelinating Disease Network. Magnetization transfer ratio recovery in new lesions decreases during adolescence in pediatric-onset multiple sclerosis patients. Neuroimage Clin. 6, 237–242 (2014).
Confavreux, C. & Vukusic, S. Natural history of multiple sclerosis: a unifying concept. Brain 129, 606–616 (2006).
Shields, S. A., Gilson, J. M., Blakemore, W. F. & Franklin, R. J. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia 28, 77–83 (1999). This article contains the first demonstration that ageing slows the process of remyelination.
Caprariello, A. V. et al. Apoptosis of oligodendrocytes during early development delays myelination and impairs subsequent responses to demyelination. J. Neurosci. 35, 14031–14041 (2015).
Shen, S. et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 11, 1024–1034 (2008).
Zhao, C., Li, W. W. & Franklin, R. J. Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-related decline in CNS remyelination. Neurobiol. Aging 27, 1298–1307 (2006).
Syed, Y. A. et al. Inhibition of oligodendrocyte precursor cell differentiation by myelin-associated proteins. Neurosurg. Focus 24, E5 (2008).
Natrajan, M. S. et al. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain 138, 3581–3597 (2015).
Syed, Y. A. et al. Antibody-mediated neutralization of myelin-associated EphrinB3 accelerates CNS remyelination. Acta Neuropathol. 131, 281–298 (2016).
Franklin, R. J. & ffrench-Constant, C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9, 839–855 (2008).
Franklin, R. J. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 3, 705–714 (2002).
Hinks, G. L. & Franklin, R. J. Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Mol. Cell. Neurosci. 16, 542–556 (2000).
Wolswijk, G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J. Neurosci. 18, 601–609 (1998). By analysing chronic demyelinated MS lesions, this study was the first to find that these lesions all contained significant numbers of OPCs, indicating that OPCs might be in a quiescent state and unable to differentiate.
Kuhlmann, T. et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131, 1749–1758 (2008).
Chang, A., Nishiyama, A., Peterson, J., Prineas, J. & Trapp, B. D. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci. 20, 6404–6412 (2000).
Wolswijk, G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain 125, 338–349 (2002).
Chang, A., Tourtellotte, W. W., Rudick, R. & Trapp, B. D. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N. Engl. J. Med. 346, 165–173 (2002).
Gautier, H. O. et al. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat. Commun. 6, 8518 (2015).
Jepson, S. et al. LINGO-1, a transmembrane signaling protein, inhibits oligodendrocyte differentiation and myelination through intercellular self-interactions. J. Biol. Chem. 287, 22184–22195 (2012).
Lee, X. et al. NGF regulates the expression of axonal LINGO-1 to inhibit oligodendrocyte differentiation and myelination. J. Neurosci. 27, 220–225 (2007).
Charles, P. et al. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc. Natl Acad. Sci. USA 97, 7585–7590 (2000).
Redmond, S. A. et al. Somatodendritic expression of JAM2 inhibits oligodendrocyte myelination. Neuron 91, 824–836 (2016).
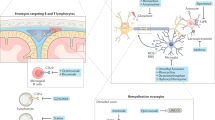
Kotter, M. R., Setzu, A., Sim, F. J., Van Rooijen, N. & Franklin, R. J. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia 35, 204–212 (2001).
Yong, V. W. & Rivest, S. Taking advantage of the systemic immune system to cure brain diseases. Neuron 64, 55–60 (2009).
Rawji, K. S. & Yong, V. W. The benefits and detriments of macrophages/microglia in models of multiple sclerosis. Clin. Dev. Immunol. 2013, 948976 (2013).
Ferguson, B., Matyszak, M. K., Esiri, M. M. & Perry, V. H. Axonal damage in acute multiple sclerosis lesions. Brain 120, 393–399 (1997).
Lucchinetti, C. et al. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain 122, 2279–2295 (1999).
Ruijs, T. C., Freedman, M. S., Grenier, Y. G., Olivier, A. & Antel, J. P. Human oligodendrocytes are susceptible to cytolysis by major histocompatibility complex class I-restricted lymphocytes. J. Neuroimmunol. 27, 89–97 (1990).
Moore, C. S. et al. Direct and indirect effects of immune and central nervous system-resident cells on human oligodendrocyte progenitor cell differentiation. J. Immunol. 194, 761–772 (2015).
El Behi, M. et al. Adaptive human immunity drives remyelination in a mouse model of demyelination. Brain 140, 967–980 (2017).
Miron, V. E. et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16, 1211–1218 (2013). By exploring pro-inflammatory and anti-inflammatory or regulatory macrophage and microglia populations during remyelination, this study demonstrates that a switch to a regulatory phenotype occurs during remyelination and promotes remyelination in part by the release of activin A.
Rinaldi, M. et al. Galectin-1 circumvents lysolecithin-induced demyelination through the modulation of microglial polarization/phagocytosis and oligodendroglial differentiation. Neurobiol. Dis. 96, 127–143 (2016).
Skihar, V. et al. Promoting oligodendrogenesis and myelin repair using the multiple sclerosis medication glatiramer acetate. Proc. Natl Acad. Sci. USA 106, 17992–17997 (2009).
Dombrowski, Y. et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 20, 674–680 (2017).
Woodruff, R. H., Fruttiger, M., Richardson, W. D. & Franklin, R. J. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol. Cell. Neurosci. 25, 252–262 (2004).
Buckley, C. E. et al. Drug reprofiling using zebrafish identifies novel compounds with potential pro-myelination effects. Neuropharmacology 59, 149–159 (2010).
Deshmukh, V. A. et al. A regenerative approach to the treatment of multiple sclerosis. Nature 502, 327–332 (2013). This study identified benztropine as a potential remyelination therapeutic that improves clinical function following autoimmune injury via remyelination and not immunomodulation.
Mei, F. et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 20, 954–960 (2014). Using an array of micropillars to screen for therapies that promote OPC differentiation, this study demonstrated that the antimuscarinic family of medications promotes oligodendrocyte differentiation and remyelination.
Najm, F. J. et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 522, 216–220 (2015). This screen identified several compounds that promote oligodendrocyte differentiation. This study converges on two potential therapies, miconazole and clobetasol, that are shown to accelerate remyelination and improve clinical scores in an autoimmune model of MS.
Joubert, L. et al. Chemical inducers and transcriptional markers of oligodendrocyte differentiation. J. Neurosci. Res. 88, 2546–2557 (2010).
Freedman, S. B., Beer, M. S. & Harley, E. A. Muscarinic M1, M2 receptor binding. Relationship with functional efficacy. Eur. J. Pharmacol. 156, 133–142 (1988).
McKearney, J. W. Stimulant actions of histamine H1 antagonists on operant behavior in the squirrel monkey. Psychopharmacology (Berl.) 77, 156–158 (1982).
Goodale, D. B. & Moore, K. E. Benztropine-induced release of dopamine from brain in vivo. Neuropharmacology 14, 585–589 (1975).
McKillop, D. & Bradford, H. F. Comparative effects of benztropine and nomifensine on dopamine uptake and release from striatal synaptosomes. Biochem. Pharmacol. 30, 2753–2758 (1981).
Abiraman, K. et al. Anti-muscarinic adjunct therapy accelerates functional human oligodendrocyte repair. J. Neurosci. 35, 3676–3688 (2015).
De Angelis, F., Bernardo, A., Magnaghi, V., Minghetti, L. & Tata, A. M. Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev. Neurobiol. 72, 713–728 (2012).
Mei, F. et al. Identification of the kappa-opioid receptor as a therapeutic target for oligodendrocyte remyelination. J. Neurosci. 36, 7925–7935 (2016).
Du, C. et al. κ opioid receptor activation alleviates experimental autoimmune encephalomyelitis and promotes oligodendrocyte-mediated remyelination. Nat. Commun. 7, 11120 (2016).
Moore, S. M. et al. Multiple functional therapeutic effects of the estrogen receptor β agonist indazole-Cl in a mouse model of multiple sclerosis. Proc. Natl Acad. Sci. USA 111, 18061–18066 (2014).
Crawford, D. K. et al. Oestrogen receptor β ligand: a novel treatment to enhance endogenous functional remyelination. Brain 133, 2999–3016 (2010).
Khalaj, A. J. et al. Estrogen receptor (ER) β expression in oligodendrocytes is required for attenuation of clinical disease by an ERβ ligand. Proc. Natl Acad. Sci. USA 110, 19125–19130 (2013).
Gonzalez, G. A. et al. Tamoxifen accelerates the repair of demyelinated lesions in the central nervous system. Sci. Rep. 6, 31599 (2016).
Franco, P. G., Silvestroff, L., Soto, E. F. & Pasquini, J. M. Thyroid hormones promote differentiation of oligodendrocyte progenitor cells and improve remyelination after cuprizone-induced demyelination. Exp. Neurol. 212, 458–467 (2008).
Harsan, L. A. et al. Recovery from chronic demyelination by thyroid hormone therapy: myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. J. Neurosci. 28, 14189–14201 (2008).
Medina-Rodriguez, E. M. et al. Promoting in vivo remyelination with small molecules: a neuroreparative pharmacological treatment for multiple sclerosis. Sci. Rep. 7, 43545 (2017).
Mi, S. et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat. Med. 13, 1228–1233 (2007).
Satoh, J., Tabunoki, H., Yamamura, T., Arima, K. & Konno, H. TROY and LINGO-1 expression in astrocytes and macrophages/microglia in multiple sclerosis lesions. Neuropathol. Appl. Neurobiol. 33, 99–107 (2007).
Koutsoudaki, P. N. et al. Remyelination after cuprizone induced demyelination is accelerated in mice deficient in the polysialic acid synthesizing enzyme St8siaIV. Neuroscience 171, 235–244 (2010).
Charles, P. et al. Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain 125, 1972–1979 (2002).
Stidworthy, M. F. et al. Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain 127, 1928–1941 (2004).
John, G. R. et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat. Med. 8, 1115–1121 (2002).
Lau, L. W., Cua, R., Keough, M. B., Haylock-Jacobs, S. & Yong, V. W. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat. Rev. Neurosci. 14, 722–729 (2013).
Sobel, R. A. & Ahmed, A. S. White matter extracellular matrix chondroitin sulfate/dermatan sulfate proteoglycans in multiple sclerosis. J. Neuropathol. Exp. Neurol. 60, 1198–1207 (2001).
Stoffels, J. M. et al. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain 136, 116–131 (2013).
Tepavcevic, V. et al. Early netrin-1 expression impairs central nervous system remyelination. Ann. Neurol. 76, 252–268 (2014).
Bin, J. M. et al. Full-length and fragmented netrin-1 in multiple sclerosis plaques are inhibitors of oligodendrocyte precursor cell migration. Am. J. Pathol. 183, 673–680 (2013).
O'Meara, R. W., Cummings, S. E., Michalski, J. P. & Kothary, R. A new in vitro mouse oligodendrocyte precursor cell migration assay reveals a role for integrin-linked kinase in cell motility. BMC Neurosci. 17, 7 (2016).
Ye, F. et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat. Neurosci. 12, 829–838 (2009).
Huang, S. M. et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620 (2009).
Preisner, A. et al. Non-steroidal anti-inflammatory drug indometacin enhances endogenous remyelination. Acta Neuropathol. 130, 247–261 (2015).
Huang, J. K. et al. Retinoid X receptor γ signaling accelerates CNS remyelination. Nat. Neurosci. 14, 45–53 (2011). By transcriptionally profiling different stages of remyelination, this study identifies RXRγ as a positive regulator of oligodendrocyte differentiation and remyelination.
de la Fuente, A. G. et al. Vitamin D receptor–retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J. Cell Biol. 211, 975–985 (2015).
Syed, Y. A. et al. Inhibition of CNS remyelination by the presence of semaphorin 3A. J. Neurosci. 31, 3719–3728 (2011).
Piaton, G. et al. Class 3 semaphorins influence oligodendrocyte precursor recruitment and remyelination in adult central nervous system. Brain 134, 1156–1167 (2011).
Giraudon, P. et al. Semaphorin CD100 from activated T lymphocytes induces process extension collapse in oligodendrocytes and death of immature neural cells. J. Immunol. 172, 1246–1255 (2004).
Tran, J. Q. et al. Randomized phase I trials of the safety/tolerability of anti-LINGO-1 monoclonal antibody BIIB033. Neurol. Neuroimmunol. Neuroinflamm. 1, e18 (2014).
Cadavid, D. et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 16, 189–199 (2017).
Cadavid, D. et al. Efficacy analysis of opicinumab in relapsing multiple sclerosis: the Phase 2b SYNERGY trial. ECTRIMS Online Library 147038 abstr. 192 (2016).
Goudie, A. J., Smith, J. A. & Millan, M. J. Characterization of the effects of receptor-selective ligands in rats discriminating the novel antipsychotic quetiapine. Psychopharmacology 171, 212–222 (2004).
Chandran, P. et al. Magnetic resonance imaging and histological evidence for the blockade of cuprizone-induced demyelination in C57BL/6 mice. Neuroscience 202, 446–453 (2012).
Zhang, Y. et al. Quetiapine alleviates the cuprizone-induced white matter pathology in the brain of C57BL/6 mouse. Schizophr. Res. 106, 182–191 (2008).
Xiao, L. et al. Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol. Psychiatry 13, 697–708 (2008).
Bordet, T. et al. Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J. Pharmacol. Exp. Ther. 322, 709–720 (2007).
Magalon, K. et al. Olesoxime accelerates myelination and promotes repair in models of demyelination. Ann. Neurol. 71, 213–226 (2012).
Pelletier, J. et al. Results of a phase 1b study to confirm safety and tolerability of olesoxime in multiple sclerosis patients [abstract]. Neurology 84 (Suppl.), P7.282 (2015).
Zhang, M., Ma, Z., Qin, H. & Yao, Z. Thyroid hormone potentially benefits multiple sclerosis via facilitating remyelination. Mol. Neurobiol. 53, 4406–4416 (2016).
Wang, R. et al. Histamine H3 receptor negatively regulates oligodendrocyte differentiation and myelination. Mult. Scler. 20 (S1), 341 https://onlinelibrary.ectrims-congress.eu/ectrims/2014/ACTRIMS-ECTRIMS2014/64324/ryan.rong.wang.histamine.h3.receptor.negatively.regulates.oligodendrocyte.html?f=m1 (2014).
Schwartzbach, C. J. et al. Lesion remyelinating activity of GSK239512 versus placebo in patients with relapsing-remitting multiple sclerosis: a randomised, single-blind, phase II study. J. Neurol. 264, 304–315 (2017).
Sedel, F., Bernard, D., Mock, D. M. & Tourbah, A. Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis. Neuropharmacology 110, 644–653 (2016).
Sedel, F. et al. High doses of biotin in chronic progressive multiple sclerosis: a pilot study. Mult. Scler. Relat. Disord. 4, 159–169 (2015).
Tourbah, A. et al. MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: a randomised, double-blind, placebo-controlled study. Mult. Scler. 22, 1719–1731 (2016).
Gregg, C. Pregnancy, prolactin and white matter regeneration. J. Neurol. Sci. 285, 22–27 (2009).
Zhornitsky, S., Johnson, T. A., Metz, L. M., Weiss, S. & Yong, V. W. Prolactin in combination with interferon-β reduces disease severity in an animal model of multiple sclerosis. J. Neuroinflamm. 12, 55 (2015).
Muraro, P. A. et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 74, 459–469 (2017).
Atkins, H. L. et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet 388, 576–585 (2016).
Copelan, E. A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 354, 1813–1826 (2006).
Brown, R. A., Narayanan, S. & Arnold, D. L. Segmentation of magnetization transfer ratio lesions for longitudinal analysis of demyelination and remyelination in multiple sclerosis. Neuroimage 66, 103–109 (2013).
Connick, P. et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 11, 150–156 (2012).
Zhang, Y. MRI texture analysis in multiple sclerosis. Int. J. Biomed. Imaging 2012, 762804 (2012).
Mathias, J. M., Tofts, P. S. & Losseff, N. A. Texture analysis of spinal cord pathology in multiple sclerosis. Magn. Reson. Med. 42, 929–935 (1999).
Fox, R. J. Picturing multiple sclerosis: conventional and diffusion tensor imaging. Semin. Neurol. 28, 453–466 (2008).
Klawiter, E. C. et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage 55, 1454–1460 (2011).
Song, S. K. et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26, 132–140 (2005).
Budde, M. D., Xie, M., Cross, A. H. & Song, S. K. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J. Neurosci. 29, 2805–2813 (2009).
Schmierer, K., Scaravilli, F., Altmann, D. R., Barker, G. J. & Miller, D. H. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann. Neurol. 56, 407–415 (2004).
Henkelman, R. M., Stanisz, G. J. & Graham, S. J. Magnetization transfer in MRI: a review. NMR Biomed. 14, 57–64 (2001).
MacKay, A. L. et al. MR relaxation in multiple sclerosis. Neuroimaging Clin. N. Am. 19, 1–26 (2009).
Laule, C. et al. Myelin water imaging of multiple sclerosis at 7 T: correlations with histopathology. Neuroimage 40, 1575–1580 (2008).
Stankoff, B. et al. Imaging central nervous system myelin by positron emission tomography in multiple sclerosis using [methyl-¹¹C]-2-(4′-methylaminophenyl)- 6-hydroxybenzothiazole. Ann. Neurol. 69, 673–680 (2011).
Schlaeger, R. et al. Monitoring multiple sclerosis by multimodal evoked potentials: numerically versus ordinally scaled scoring systems. Clin. Neurophysiol. 127, 1864–1871 (2016).
Ontaneda, D. & Fox, R. J. Imaging as an outcome measure in multiple sclerosis. Neurotherapeutics 14, 24–34 (2017).
Aharoni, R. et al. Assessing remyelination — metabolic labeling of myelin in an animal model of multiple sclerosis. J. Neuroimmunol. 301, 7–11 (2016).
Yazdi, A., Baharvand, H. & Javan, M. Enhanced remyelination following lysolecithin-induced demyelination in mice under treatment with fingolimod (FTY720). Neuroscience 311, 34–44 (2015).
Blakemore, W. F. Ethidium bromide induced demyelination in the spinal cord of the cat. Neuropathol. Appl. Neurobiol. 8, 365–375 (1982).
Blakemore, W. F. The response of oligodendrocytes to chemical injury. Acta Neurol. Scand. Suppl. 100, 33–38 (1984).
Blakemore, W. F. The case for a central nervous system (CNS) origin for the Schwann cells that remyelinate CNS axons following concurrent loss of oligodendrocytes and astrocytes. Neuropathol. Appl. Neurobiol. 31, 1–10 (2005).
Zawadzka, M. et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6, 578–590 (2010).
Matsushima, G. K. & Morell, P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 11, 107–116 (2001).
Sturrock, R. R. Myelination of the mouse corpus callosum. Neuropathol. Appl. Neurobiol. 6, 415–420 (1980).
Denic, A. et al. The relevance of animal models in multiple sclerosis research. Pathophysiology 18, 21–29 (2011).
Rodriguez, M., Oleszak, E. & Leibowitz, J. Theiler's murine encephalomyelitis: a model of demyelination and persistence of virus. Crit. Rev. Immunol. 7, 325–365 (1987).
Dal Canto, M. C. & Lipton, H. L. Multiple sclerosis. Animal model:Theiler's virus infection in mice. Am. J. Pathol. 88, 497–500 (1977).
Bieber, A. J., Ure, D. R. & Rodriguez, M. Genetically dominant spinal cord repair in a murine model of chronic progressive multiple sclerosis. J. Neuropathol. Exp. Neurol. 64, 46–57 (2005).
Zhang, Y. et al. Multi-scale MRI spectrum detects differences in myelin integrity between MS lesion types. Mult. Scler. 22, 1569–1577 (2016).
Schmierer, K. et al. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J. Magn. Reson. Imaging 26, 41–51 (2007).
Acknowledgements
The authors' research on remyelination has been funded by operating grants from the Alberta Innovates Health Solutions CRIO Team Program, the Multiple Sclerosis Society of Canada and the Canadian Institutes of Health Research. The authors thank A. Caprariello, S. Jensen, M. Keough, G. Duncan and K. Rawji for critically reading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Relapsing–remitting MS
-
A form of multiple sclerosis (MS) that is characterized by episodes or attacks, called relapses, which cause neurologic dysfunction with variable recovery over time, interspersed with periods of stability (also known as remission).
- Progressive MS
-
A form of multiple sclerosis (MS) that is characterized by a slow accumulation of disability without relapses. It is classified as primary progressive MS if patients present with a progressive disease course from onset and secondary progressive MS if patients initially present with a course of relapsing–remitting MS and later convert to progressive disease.
- Oligodendrocyte
-
A type of neuroglial cell that produces myelin.
- Compact myelin
-
In the central nervous system, the wrapping of multiple layers of oligodendrocyte membrane forms myelin. In its mature form, these membrane layers become compact.
- Nodes of Ranvier
-
Gaps between two adjacent myelinated regions where the axon is exposed to the extracellular space.
- Oligodendrocyte progenitor cells
-
Cells that express the characteristic markers platelet-derived growth factor receptor-α (PDGFRα) and chondroitin sulfate proteoglycan 4 (CSPG4, also known as NG2), and that mature into oligodendrocytes during development and remyelination. These cells are also referred to as polydendrocytes or NG2 cells.
- White matter
-
Areas of the central nervous system containing primarily myelinated axons. Owing to the lipid-rich composition of myelin, these areas appear white compared with adjacent areas.
- Grey matter
-
Areas of the central nervous system containing less myelination; it is enriched with neuronal cell bodies and dendrites.
- Visual evoked potential
-
The measured surface electrical activity in the brain in response to visual stimulation.
Rights and permissions
About this article
Cite this article
Plemel, J., Liu, WQ. & Yong, V. Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat Rev Drug Discov 16, 617–634 (2017). https://doi.org/10.1038/nrd.2017.115
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2017.115
This article is cited by
-
Interplay between androgen and CXCR4 chemokine signaling in myelin repair
Acta Neuropathologica Communications (2024)
-
Mitochondrial and metabolic dysfunction of peripheral immune cells in multiple sclerosis
Journal of Neuroinflammation (2024)
-
Unravelling the Road to Recovery: Mechanisms of Wnt Signalling in Spinal Cord Injury
Molecular Neurobiology (2024)
-
Interleukin-6 Inhibits Expression of miR-204-5p, a Regulator of Oligodendrocyte Differentiation: Involvement of miR-204-5p in the Prevention of Chemical-Induced Oligodendrocyte Impairment
Molecular Neurobiology (2024)
-
Regulatory T cells alleviate myelin loss and cognitive dysfunction by regulating neuroinflammation and microglial pyroptosis via TLR4/MyD88/NF-κB pathway in LPC-induced demyelination
Journal of Neuroinflammation (2023)