Key Points
-
Type 2 diabetes is characterized by elevated blood glucose levels and insulin resistance. Current diabetes drugs can lower blood glucose but often have side effects, and the most widely used drug — metformin — does not have a clear mechanism of action.
-
Targeting glucose and glycogen metabolism in the liver is a strategy for type 2 diabetes treatment, as it can decrease hepatic glucose output, but this approach has not been fully explored.
-
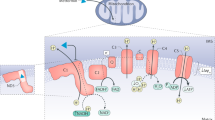
Gluconeogenic and glycogenolytic enzymes or their regulators present numerous drug targets that are currently being investigated or have the potential to be developed.
-
Transcriptional co-activators and transcription factors are emerging diabetes drug targets with the ability to control entire gene programmes involved in glucose and glycogen metabolism. It may be possible to specifically target these transcriptional regulators by modulating their protein–protein interactions or post-translational modifications.
-
Novel diabetes drugs would most probably be used in combination with existing therapies to enable sustained blood glucose suppression, and so that each drug could be used at a lower concentration to limit side effects. Drugs decreasing hepatic glucose output may be most effectively used with drugs that work by other mechanisms, such as thiazolidinediones or sodium-glucose co-transporter 2 (SGLT2) inhibitors.
-
Challenges of inhibiting hepatic glucose output include preventing hypoglycaemia, enabling tissue-specific targeting, analysing the possible effects of redirecting carbons to triglyceride or cholesterol synthesis, and avoiding lactic acidosis.
Abstract
Type 2 diabetes mellitus is characterized by the dysregulation of glucose homeostasis, resulting in hyperglycaemia. Although current diabetes treatments have exhibited some success in lowering blood glucose levels, their effect is not always sustained and their use may be associated with undesirable side effects, such as hypoglycaemia. Novel antidiabetic drugs, which may be used in combination with existing therapies, are therefore needed. The potential of specifically targeting the liver to normalize blood glucose levels has not been fully exploited. Here, we review the molecular mechanisms controlling hepatic gluconeogenesis and glycogen storage, and assess the prospect of therapeutically targeting associated pathways to treat type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States, 2014 https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf (US Department of Health and Human Services, 2014).
Hossain, P., Kawar, B. & El Nahas, M. Obesity and diabetes in the developing world — a growing challenge. N. Engl. J. Med. 356, 213–215 (2007).
DeFronzo, R. A., Bonadonna, R. C. & Ferrannini, E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care 15, 318–368 (1992).
Foretz, M., Guigas, B., Bertrand, L., Pollak, M. & Viollet, B. Metformin: from mechanisms of action to therapies. Cell. Metab. 20, 953–966 (2014). This work reviews the complexities surrounding the mechanism of action of metformin, the most widely used antidiabetic drug.
Gribble, F. M. & Reimann, F. Sulphonylurea action revisited: the post-cloning era. Diabetologia 46, 875–891 (2003).
Drucker, D. J. & Nauck, M. A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705 (2006).
Soccio, R. E., Chen, E. R. & Lazar, M. A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell. Metab. 20, 573–591 (2014).
Derosa, G. & Maffioli, P. Efficacy and safety profile evaluation of acarbose alone and in association with other antidiabetic drugs: a systematic review. Clin. Ther. 34, 1221–1236 (2012).
Chao, E. C. & Henry, R. R. SGLT2 inhibition — a novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 9, 551–559 (2010).
Handelsman, Y. Role of bile acid sequestrants in the treatment of type 2 diabetes. Diabetes Care 34 (Suppl. 2), 244–250 (2011).
Bouchoucha, M., Uzzan, B. & Cohen, R. Metformin and digestive disorders. Diabetes Metab. 37, 90–96 (2011).
Del Prato, S. & Pulizzi, N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metabolism 55, S20–S27 (2006).
Despres, J. P. et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N. Engl. J. Med. 334, 952–957 (1996).
Gallagher, E. J. & LeRoith, D. Epidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancer. Diabetes Care 36 (Suppl. 2), 233–239 (2013).
Postic, C., Dentin, R. & Girard, J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 30, 398–408 (2004).
Meyer, C., Dostou, J. M., Welle, S. L. & Gerich, J. E. Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 282, E419–E427 (2002).
Kiyosue, A., Hayashi, N., Komori, H., Leonsson-Zachrisson, M. & Johnsson, E. Dose-ranging study with the glucokinase activator AZD1656 as monotherapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 15, 923–930 (2013).
Matschinsky, F. M. Assessing the potential of glucokinase activators in diabetes therapy. Nat. Rev. Drug Discov. 8, 399–416 (2009). This review discusses the development of glucokinase activators as drugs for type 2 diabetes.
Erion, D. M. et al. The hepatoselective glucokinase activator PF-04991532 ameliorates hyperglycemia without causing hepatic steatosis in diabetic rats. PloS ONE 9, e97139 (2014).
Sharma, R. et al. Comparison of the circulating metabolite profile of PF-04991532, a hepatoselective glucokinase activator, across preclinical species and humans: potential implications in metabolites in safety testing assessment. Drug Metab. Dispos. 43, 190–198 (2015).
Katz, L. et al. AMG 151 (ARRY-403), a novel glucokinase activator, decreases fasting and postprandial glycaemia in patients with type 2 diabetes. Diabetes Obes. Metab. 18, 191–195 (2016).
Zhi, J. & Zhai, S. Effects of piragliatin, a glucokinase activator, on fasting and postprandial plasma glucose in patients with type 2 diabetes mellitus. J. Clin. Pharmacol. 56, 231–238 (2016).
Lloyd, D. J. et al. Antidiabetic effects of glucokinase regulatory protein small-molecule disruptors. Nature 504, 437–440 (2013). The authors identify two small-molecule inhibitors of the glucokinase–GKRP interaction, and their ability to lower blood glucose levels in diabetic rodents.
Valcarce, C. The importance of tissue selectivity and preservation of the physiological regulation when targeting key metabolic regulators as glucokinase. Keystone Symposia on New Therapeutics for Diabetes and Obesity, http://vtvtherapeutics.com/assets/docs/TTP399_Presentation_Keystone_Symposia_2016-FINAL.pdf (2016).
Agius, L. New hepatic targets for glycaemic control in diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 21, 587–605 (2007).
Oikonomakos, N. G. Glycogen phosphorylase as a molecular target for type 2 diabetes therapy. Curr. Protein Pept. Sci. 3, 561–586 (2002).
Treadway, J. L., Mendys, P. & Hoover, D. J. Glycogen phosphorylase inhibitors for treatment of type 2 diabetes mellitus. Expert Opin. Investigat. Drugs 10, 439–454 (2001).
Ogawa, A. K. et al. Glucose-lowering in a db/db mouse model by dihydropyridine diacid glycogen phosphorylase inhibitors. Bioorg. Med. Chem. Lett. 13, 3405–3408 (2003).
Martin, W. H. et al. Discovery of a human liver glycogen phosphorylase inhibitor that lowers blood glucose in vivo. Proc. Natl Acad. Sci. USA 95, 1776–1781 (1998).
Rath, V. L. et al. Human liver glycogen phosphorylase inhibitors bind at a new allosteric site. Chem. Biol. 7, 677–682 (2000).
Furukawa, S., Murakami, K., Nishikawa, M., Nakayama, O. & Hino, M. FR258900, a novel glycogen phosphorylase inhibitor isolated from Fungus No. 138354. II. Anti-hyperglycemic effects in diabetic animal models. J. Antibiot. 58, 503–506 (2005).
Suh, S. W. et al. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmethyl)pro pyl]-1H-indole-2-carboxamide). J. Pharmacol. Exp. Ther. 321, 45–50 (2007).
Torres, T. P. et al. Impact of a glycogen phosphorylase inhibitor and metformin on basal and glucagon-stimulated hepatic glucose flux in conscious dogs. J. Pharmacol. Exp. Ther. 337, 610–620 (2011).
Flattem, N. et al. α- and β-cell responses to small changes in plasma glucose in the conscious dog. Diabetes 50, 367–375 (2001).
Kazierad, D. J. et al. Effects of multiple ascending doses of the glucagon receptor antagonist, PF-06291874, in patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 18, 795–802 (2016).
Vajda, E. J. et al. Pharmacodynamic effects of single doses of the glucagon receptor antagonist LGD-6972 in healthy subjects and subjects with type 2 diabetes mellitus (T2DM). Endocrine Society's 97th Annual Meeting and Expo, http://press.endocrine.org/doi/abs/10.1210/endo-meetings.2015.DGM.2.PP01-1 (2015).
Petersen, K. F. & Sullivan, J. T. Effects of a novel glucagon receptor antagonist (Bay 27–9955) on glucagon-stimulated glucose production in humans. Diabetologia 44, 2018–2024 (2001).
Guan, H. P. et al. Glucagon receptor antagonism induces increased cholesterol absorption. J. Lipid Res. 56, 2183–2195 (2015).
Xiong, Y. et al. Discovery of a novel glucagon receptor antagonist N-[(4-{(1S)-1-[3-(3, 5-dichlorophenyl)-5-(6-methoxynaphthalen-2-yl)-1H-pyrazol-1-yl]ethyl}phenyl)carbo nyl]-β-alanine (MK-0893) for the treatment of type II diabetes. J. Med. Chem. 55, 6137–6148 (2012).
Kelly, R. P. et al. Short-term administration of the glucagon receptor antagonist LY2409021 lowers blood glucose in healthy people and in those with type 2 diabetes. Diabetes Obes. Metab. 17, 414–422 (2015).
Kazda, C. M. et al. A randomized, double-blind, placebo-controlled phase 2 study of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes. Diabetes Care 39, 1241–1249 (2015).
van Poelje, P. D. et al. Inhibition of fructose 1,6-bisphosphatase reduces excessive endogenous glucose production and attenuates hyperglycemia in Zucker diabetic fatty rats. Diabetes 55, 1747–1754 (2006).
Dang, Q. et al. Fructose-1,6-bisphosphatase Inhibitors. 2. Design, synthesis, and structure-activity relationship of a series of phosphonic acid containing benzimidazoles that function as 5′-adenosinemonophosphate (AMP) mimics. J. Med. Chem. 53, 441–451 (2010).
Aicher, T. D., Boyd, S. A., McVean, M. & Celeste, A. Novel therapeutics and targets for the treatment of diabetes. Expert Rev. Clin. Pharmacol. 3, 209–229 (2010).
Liu, G. Technology evaluation: ISIS-113715, Isis. Curr. Opin. Mol. Ther. 6, 331–336 (2004).
Rondinone, C. M. et al. Protein tyrosine phosphatase 1B reduction regulates adiposity and expression of genes involved in lipogenesis. Diabetes 51, 2405–2411 (2002).
Swarbrick, M. M. et al. Inhibition of protein tyrosine phosphatase-1B with antisense oligonucleotides improves insulin sensitivity and increases adiponectin concentrations in monkeys. Endocrinology 150, 1670–1679 (2009).
Wrobel, J. et al. PTP1B inhibition and antihyperglycemic activity in the ob/ob mouse model of novel 11-arylbenzo[b]naphtho[2,3-d]furans and 11-arylbenzo[b]naphtho[2,3-d]thiophenes. J. Med. Chem. 42, 3199–3202 (1999).
Erbe, D. V. et al. Ertiprotafib improves glycemic control and lowers lipids via multiple mechanisms. Mol. Pharmacol. 67, 69–77 (2005).
Shrestha, S., Bhattarai, B. R., Cho, H., Choi, J. K. & Cho, H. PTP1B inhibitor Ertiprotafib is also a potent inhibitor of IκB kinase β (IKK-β). Bioorg. Med. Chem. Lett. 17, 2728–2730 (2007).
Zasloff, M. et al. A spermine-coupled cholesterol metabolite from the shark with potent appetite suppressant and antidiabetic properties. Int. J. Obes. Relat. Metabol. Disord. 25, 689–697 (2001).
Lantz, K. A. et al. Inhibition of PTP1B by trodusquemine (MSI-1436) causes fat-specific weight loss in diet-induced obese mice. Obesity 18, 1516–1523 (2010).
Cho, H. Protein tyrosine phosphatase 1B (PTP1B) and obesity. Vitam. Horm. 91, 405–424 (2013).
Krishnan, N. et al. Targeting the disordered C terminus of PTP1B with an allosteric inhibitor. Nat. Chem. Biol. 10, 558–566 (2014).
Bano, G. Glucose homeostasis, obesity and diabetes. Best Pract. Res. Clin. Obstetr. Gynaecol. 27, 715–726 (2013).
Cano, N. Bench-to-bedside review: glucose production from the kidney. Crit. Care 6, 317–321 (2002).
Mithieux, G., Andreelli, F. & Magnan, C. Intestinal gluconeogenesis: key signal of central control of energy and glucose homeostasis. Curr. Opin. Clin. Nutr. Metabol. Care 12, 419–423 (2009).
Radziuk, J. & Pye, S. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabetes Metab. Res. Rev. 17, 250–272 (2001).
Taylor, S. I. Deconstructing type 2 diabetes. Cell 97, 9–12 (1999).
Newgard, C. B., Hirsch, L. J., Foster, D. W. & McGarry, J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J. Biol. Chem. 258, 8046–8052 (1983).
Moore, M. C. et al. Sources of carbon for hepatic glycogen synthesis in the conscious dog. J. Clin. Invest. 88, 578–587 (1991).
Edgerton, D. S. et al. Insulin's direct effects on the liver dominate the control of hepatic glucose production. J. Clin. Invest. 116, 521–527 (2006). This study utilizes a normal dog model to demonstrate that the direct effects of insulin predominately regulate glucose production in the liver, whereas insulin action at the hypothalamus has negligible effects.
Thiebaud, D. et al. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes 31, 957–963 (1982).
Dobbins, R. L. et al. Role of glucagon in countering hypoglycemia induced by insulin infusion in dogs. Am. J. Physiol. 261, E773–E781 (1991).
Landau, B. R. et al. Contributions of gluconeogenesis to glucose production in the fasted state. J. Clin. Invest. 98, 378–385 (1996). This study examined healthy human subjects to conclude that gluconeogenesis contributes to 50% of glucose production after an overnight fast, and to nearly all of glucose production within the following 2 days.
Rothman, D. L., Magnusson, I., Katz, L. D., Shulman, R. G. & Shulman, G. I. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science 254, 573–576 (1991).
Weber, G., Singhal, R. L., Stamm, N. B., Fisher, E. A. & Mentendiek, M. A. Regulation of enzymes involved in gluconeogenesis. Adv. Enzyme Regul. 2, 1–38 (1964).
Abdul-Ghani, M. A., Tripathy, D. & DeFronzo, R. A. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29, 1130–1139 (2006).
Kahn, S. E., Cooper, M. E. & Del Prato, S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383, 1068–1083 (2014).
D'Alessio, D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes. Metab. 13 (Suppl. 1), 126–132 (2011).
Magnusson, I., Rothman, D. L., Katz, L. D., Shulman, R. G. & Shulman, G. I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J. Clin. Invest. 90, 1323–1327 (1992).
Wajngot, A. et al. Quantitative contributions of gluconeogenesis to glucose production during fasting in type 2 diabetes mellitus. Metabolism 50, 47–52 (2001).
Hwang, J. H. et al. Impaired net hepatic glycogen synthesis in insulin-dependent diabetic subjects during mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy study. J. Clin. Invest. 95, 783–787 (1995).
Calcutt, N. A., Cooper, M. E., Kern, T. S. & Schmidt, A. M. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat. Rev. Drug Discov. 8, 417–429 (2009).
Turner, R. C., Cull, C. A., Frighi, V. & Holman, R. R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 281, 2005–2012 (1999).
Pfefferkorn, J. A. et al. Discovery of (S)-6-(3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanamido)nicotini c acid as a hepatoselective glucokinase activator clinical candidate for treating type 2 diabetes mellitus. J. Med. Chem. 55, 1318–1333 (2012).
Taniguchi, C. M., Emanuelli, B. & Kahn, C. R. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 (2006).
Cusi, K. et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Invest. 105, 311–320 (2000).
Elchebly, M. et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283, 1544–1548 (1999). The authors of this study use knockout mice to demonstrate that PTP1B lowers blood glucose levels, enhances insulin sensitivity and provides resistance to weight gain, identifying PTP1B as a potential target for type 2 diabetes treatment.
Delibegovic, M. et al. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes 58, 590–599 (2009).
Owen, C. et al. Inducible liver-specific knockdown of protein tyrosine phosphatase 1B improves glucose and lipid homeostasis in adult mice. Diabetologia 56, 2286–2296 (2013).
Chen, P. J., Cai, S. P., Huang, C., Meng, X. M. & Li, J. Protein tyrosine phosphatase 1B (PTP1B): a key regulator and therapeutic target in liver diseases. Toxicology 337, 10–20 (2015).
Krssak, M. et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 53, 3048–3056 (2004).
Cline, G. W. et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N. Engl. J. Med. 341, 240–246 (1999).
Samuel, V. T. & Shulman, G. I. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Invest. 126, 12–22 (2016).
Furukawa, S. et al. FR258900, a novel glycogen phosphorylase inhibitor isolated from Fungus No. 138354. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 58, 497–502 (2005).
Tiraidis, C. et al. FR258900, a potential anti-hyperglycemic drug, binds at the allosteric site of glycogen phosphorylase. Protein Sci. 16, 1773–1782 (2007).
Somsak, L. et al. New inhibitors of glycogen phosphorylase as potential antidiabetic agents. Curr. Med. Chem. 15, 2933–2983 (2008).
Baker, D. J., Timmons, J. A. & Greenhaff, P. L. Glycogen phosphorylase inhibition in type 2 diabetes therapy: a systematic evaluation of metabolic and functional effects in rat skeletal muscle. Diabetes 54, 2453–2459 (2005).
Lu, Z. et al. A new class of glycogen phosphorylase inhibitors. Bioorg. Med. Chem. Lett. 13, 4125–4128 (2003).
Tracey, W. R. et al. Cardioprotective effects of ingliforib, a novel glycogen phosphorylase inhibitor. Am. J. Physiol. Heart Circ. Physiol. 286, H1177–H1184 (2004).
Hers, H. G. The control of glycogen metabolism in the liver. Annu. Rev. Biochem. 45, 167–189 (1976).
Hutson, N. J., Brumley, F. T., Assimacopoulos, F. D., Harper, S. C. & Exton, J. H. Studies on the α-adrenergic activation of hepatic glucose output. I. Studies on the α-adrenergic activation of phosphorylase and gluconeogenesis and inactivation of glycogen synthase in isolated rat liver parenchymal cells. J. Biol. Chem. 251, 5200–5208 (1976).
Embi, N., Rylatt, D. B. & Cohen, P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. FEBS 107, 519–527 (1980).
Nikoulina, S. E. et al. Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes 49, 263–271 (2000).
Eldar-Finkelman, H., Schreyer, S. A., Shinohara, M. M., LeBoeuf, R. C. & Krebs, E. G. Increased glycogen synthase kinase-3 activity in diabetes- and obesity-prone C57BL/6J mice. Diabetes 48, 1662–1666 (1999).
Coghlan, M. P. et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 7, 793–803 (2000).
Lochhead, P. A., Coghlan, M., Rice, S. Q. & Sutherland, C. Inhibition of GSK-3 selectively reduces glucose-6-phosphatase and phosphatase and phosphoenolypyruvate carboxykinase gene expression. Diabetes 50, 937–946 (2001).
Cohen, P. & Goedert, M. GSK3 inhibitors: development and therapeutic potential. Nat. Rev. Drug Discov. 3, 479–487 (2004). This review discusses the biology of GSK3 inhibitors and their development as a treatment for type 2 diabetes.
Cline, G. W. et al. Effects of a novel glycogen synthase kinase-3 inhibitor on insulin-stimulated glucose metabolism in Zucker diabetic fatty (fa/fa) rats. Diabetes 51, 2903–2910 (2002).
Ring, D. B. et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes 52, 588–595 (2003).
Kaidanovich-Beilin, O. & Eldar-Finkelman, H. Long-term treatment with novel glycogen synthase kinase-3 inhibitor improves glucose homeostasis in ob/ob mice: molecular characterization in liver and muscle. J. Pharmacol. Exp. Ther. 316, 17–24 (2006).
Eldar-Finkelman, H. & Martinez, A. GSK-3 inhibitors: preclinical and clinical focus on CNS. Frontiers Mol. Neurosci. 4, 32 (2011).
Gomez-Valades, A. G. et al. Overcoming diabetes-induced hyperglycemia through inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP) with RNAi. Mol. Ther. 13, 401–410 (2006).
Georgievska, B. et al. AZD1080, a novel GSK3 inhibitor, rescues synaptic plasticity deficits in rodent brain and exhibits peripheral target engagement in humans. J. Neurochem. 125, 446–456 (2013).
Tolosa, E. et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov. Disord. 29, 470–478 (2014).
Dominguez, J. M. et al. Evidence for irreversible inhibition of glycogen synthase kinase-3β by tideglusib. J. Biol. Chem. 287, 893–904 (2012).
Baron, A. D., Schaeffer, L., Shragg, P. & Kolterman, O. G. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36, 274–283 (1987).
Matsuda, M. et al. Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism 51, 1111–1119 (2002).
Sorensen, H. et al. Immunoneutralization of endogenous glucagon reduces hepatic glucose output and improves long-term glycemic control in diabetic ob/ob mice. Diabetes 55, 2843–2848 (2006).
O'Harte, F. P., Franklin, Z. J. & Irwin, N. Two novel glucagon receptor antagonists prove effective therapeutic agents in high-fat-fed and obese diabetic mice. Diabetes Obes. Metab. 16, 1214–1222 (2014).
Qureshi, S. A. et al. A novel glucagon receptor antagonist inhibits glucagon-mediated biological effects. Diabetes 53, 3267–3273 (2004).
Rivera, N. et al. A novel glucagon receptor antagonist, NNC 25–0926, blunts hepatic glucose production in the conscious dog. J. Pharmacol. Exp. Ther. 321, 743–752 (2007).
Perry, R. J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015). The authors of this study discovered that insulin can decrease hepatic gluconeogenesis and protect against hyperglycaemia induced by a high-fat diet through the inhibition of lipolysis in white adipose tissue, which decreases acetyl CoA and pyruvate carboxylase activity in the liver.
Kumashiro, N. et al. Targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes 62, 2183–2194 (2013).
Bahl, J. J., Matsuda, M., DeFronzo, R. A. & Bressler, R. In vitro and in vivo suppression of gluconeogenesis by inhibition of pyruvate carboxylase. Biochem. Pharmacol. 53, 67–74 (1997).
Farfari, S., Schulz, V., Corkey, B. & Prentki, M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 49, 718–726 (2000).
McCommis, K. S. & Finck, B. N. Mitochondrial pyruvate transport: a historical perspective and future research directions. Biochem. J. 466, 443–454 (2015).
Bricker, D. K. et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast. Drosophila, and humans. Science 337, 96–100 (2012).
Herzig, S. et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science 337, 93–96 (2012).
Gray, L. R. et al. Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole-body glucose homeostasis. Cell. Metab. 22, 669–681 (2015). This work demonstrates that the mitochondrial pyruvate carrier contributes significantly to pyruvate-driven gluconeogenesis and hyperglycaemia in type 2 diabetes.
McCommis, K. S. et al. Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell. Metab. 22, 682–694 (2015).
Divakaruni, A. S. et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc. Natl Acad. Sci. USA 110, 5422–5427 (2013).
Colca, J. R. et al. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT)—relationship to newly identified mitochondrial pyruvate carrier proteins. PloS ONE 8, e61551 (2013).
Vacanti, N. M. et al. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol. Cell 56, 425–435 (2014).
Barthel, A. & Schmoll, D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 285, E685–E692 (2003).
Cimbala, M. A. et al. Rapid changes in the concentration of phosphoenolpyruvate carboxykinase mRNA in rat liver and kidney. Effects of insulin and cyclic AMP. J. Biol. Chem. 257, 7629–7636 (1982).
Granner, D., Andreone, T., Sasaki, K. & Beale, E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature 305, 549–551 (1983).
Magnuson, M. A., Quinn, P. G. & Granner, D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase-chloramphenicol acetyltransferase fusion genes. Insulin's effects oppose those of cAMP and dexamethasone. J. Biol. Chem. 262, 14917–14920 (1987).
Valera, A., Pujol, A., Pelegrin, M. & Bosch, F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc. Natl Acad. Sci. USA 91, 9151–9154 (1994).
DiTullio, N. W. et al. 3-mercaptopicolinic acid, an inhibitor of gluconeogenesis. Biochem. J. 138, 387–394 (1974).
Samuel, V. T. et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc. Natl Acad. Sci. USA 106, 12121–12126 (2009).
Hakimi, P. et al. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr. Metab. 2, 33 (2005).
She, P. et al. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol. Cell. Biol. 20, 6508–6517 (2000).
Burgess, S. C. et al. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J. Biol. Chem. 279, 48941–48949 (2004).
Burgess, S. C. et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell. Metab. 5, 313–320 (2007). The authors of this study found that a 90% reduction in hepatic PEPCK in mice results in only a 40% decrease in gluconeogenic flux, demonstrating that the effect of PEPCK alone on gluconeogenesis is not as strong as expected, and that PEPCK may need to function together with hepatic energy production to control gluconeogenesis.
Mutel, E. et al. Control of blood glucose in the absence of hepatic glucose production during prolonged fasting in mice: induction of renal and intestinal gluconeogenesis by glucagon. Diabetes 60, 3121–3131 (2011).
Abdul-Wahed, A. et al. A link between hepatic glucose production and peripheral energy metabolism via hepatokines. Mol. Metab. 3, 531–543 (2014).
Rhee, J. et al. Regulation of hepatic fasting response by PPARγ coactivator-1α (PGC-1): requirement for hepatocyte nuclear factor 4α in gluconeogenesis. Proc. Natl Acad. Sci. USA 100, 4012–4017 (2003).
Codogno, P. & Meijer, A. J. Autophagy in the liver. J. Hepatol. 59, 389–391 (2013).
Okar, D. A. & Lange, A. J. Fructose-2,6-bisphosphate and control of carbohydrate metabolism in eukaryotes. BioFactors 10, 1–14 (1999).
Wu, C., Okar, D. A., Newgard, C. B. & Lange, A. J. Increasing fructose 2,6-bisphosphate overcomes hepatic insulin resistance of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 282, E38–E45 (2002).
Wu, C., Okar, D. A., Newgard, C. B. & Lange, A. J. Overexpression of 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase in mouse liver lowers blood glucose by suppressing hepatic glucose production. J. Clin. Invest. 107, 91–98 (2001). This study overexpressed PFK/FBPase 2 in normal and diabetic mice, which resulted in decreased blood glucose levels and hepatic glucose production; these findings suggest that this enzyme may be a target for type 2 diabetes treatment.
Cochran, A. G. Antagonists of protein-protein interactions. Chem. Biol. 7, R85–R94 (2000).
Song, X. et al. Development of potent small-molecule inhibitors to drug the undruggable steroid receptor coactivator-3. Proc. Natl Acad. Sci. USA 113, 4970–4975 (2016).
Fontaine, F., Overman, J. & Francois, M. Pharmacological manipulation of transcription factor protein-protein interactions: opportunities and obstacles. Cell Regener. 4, 2 (2015).
Thompson, A. D., Dugan, A., Gestwicki, J. E. & Mapp, A. K. Fine-tuning multiprotein complexes using small molecules. ACS Chem. Biol. 7, 1311–1320 (2012).
Bhagwat, A. S. & Vakoc, C. R. Targeting transcription factors in cancer. Trends Cancer 1, 53–65 (2015).
Darnell, J. E. Jr Transcription factors as targets for cancer therapy. Nat. Rev. Cancer 2, 740–749 (2002).
Liang, H. & Ward, W. F. PGC-1α: a key regulator of energy metabolism. Adv. Physiol. Educ. 30, 145–151 (2006).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Esterbauer, H., Oberkofler, H., Krempler, F. & Patsch, W. Human peroxisome proliferator activated receptor gamma coactivator 1 (PPARGC1) gene: cDNA sequence, genomic organization, chromosomal localization, and tissue expression. Genomics 62, 98–102 (1999).
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999).
Yoon, J. C. et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413, 131–138 (2001). The authors show that PGC1α is strongly induced by fasting in mouse livers, and it also activates the gluconeogenic programme and glucose output in hepatocytes.
Puigserver, P. et al. Insulin-regulated hepatic gluconeogenesis through FOXO1–PGC-1α interaction. Nature 423, 550–555 (2003). This work demonstrates that FOXO1 is required for PGC1α-mediated activation of the insulin-regulated gluconeogenic gene programme.
Koo, S. H. et al. PGC-1 promotes insulin resistance in liver through PPAR-α-dependent induction of TRB-3. Nat. Med. 10, 530–534 (2004).
Leone, T. C. et al. PGC-1α deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 3, e101 (2005).
Lin, J. et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell 119, 121–135 (2004).
Estall, J. L. et al. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-γ coactivator-1α expression. Diabetes 58, 1499–1508 (2009).
Burgess, S. C. et al. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α)-deficient mice. J. Biol. Chem. 281, 19000–19008 (2006).
Li, X., Monks, B., Ge, Q. & Birnbaum, M. J. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1α transcription coactivator. Nature 447, 1012–1016 (2007).
Rodgers, J. T., Haas, W., Gygi, S. P. & Puigserver, P. Cdc2-like kinase 2 is an insulin-regulated suppressor of hepatic gluconeogenesis. Cell. Metab. 11, 23–34 (2010).
Dominy, J. E. Jr., Lee, Y., Gerhart-Hines, Z. & Puigserver, P. Nutrient-dependent regulation of PGC-1α's acetylation state and metabolic function through the enzymatic activities of Sirt1/GCN5. Biochim. Biophys. Acta 1804, 1676–1683 (2010).
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005).
Lerin, C. et al. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1α. Cell. Metab. 3, 429–438 (2006).
Dominy, J. E. Jr. et al. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Mol. Cell 48, 900–913 (2012).
Lee, Y. et al. Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression. Nature 510, 547–551 (2014).
Teyssier, C., Ma, H., Emter, R., Kralli, A. & Stallcup, M. R. Activation of nuclear receptor coactivator PGC-1α by arginine methylation. Genes Dev. 19, 1466–1473 (2005).
Lustig, Y. et al. Separation of the gluconeogenic and mitochondrial functions of PGC-1α through S6 kinase. Genes Dev. 25, 1232–1244 (2011).
Sakai, M. et al. CITED2 links hormonal signaling to PGC-1α acetylation in the regulation of gluconeogenesis. Nat. Med. 18, 612–617 (2012).
Dekker, F. J., van den Bosch, T. & Martin, N. I. Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases. Drug Discov. Today 19, 654–660 (2014).
Brooks, C. L. & Gu, W. How does SIRT1 affect metabolism, senescence and cancer? Nat. Rev. Cancer 9, 123–128 (2009).
Mihaylova, M. M. & Shaw, R. J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 13, 1016–1023 (2011).
Chang, H. C. & Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 25, 138–145 (2014).
Ruderman, N. B., Carling, D., Prentki, M. & Cacicedo, J. M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 123, 2764–2772 (2013).
Arany, Z. et al. Gene expression-based screening identifies microtubule inhibitors as inducers of PGC-1α and oxidative phosphorylation. Proc. Natl Acad. Sci. USA 105, 4721–4726 (2008).
Zhang, L. N. et al. Novel small-molecule PGC-1α transcriptional regulator with beneficial effects on diabetic db/db mice. Diabetes 62, 1297–1307 (2013).
Burgering, B. M. A brief introduction to FOXOlogy. Oncogene 27, 2258–2262 (2008).
Haeusler, R. A., Kaestner, K. H. & Accili, D. FoxOs function synergistically to promote glucose production. J. Biol. Chem. 285, 35245–35248 (2010). This work demonstrates that ablation of hepatic FOXO2 and FOXO3 with FOXO1 lowers blood glucose levels and increases insulin sensitivity to a greater extent than loss of FOXO1 alone, which suggests that FOXO isoforms function together to promote hepatic gluconeogenesis.
Kim, D. H. et al. FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes 60, 2763–2774 (2011).
Tikhanovich, I., Cox, J. & Weinman, S. A. Forkhead box class O transcription factors in liver function and disease. J. Gastroenterol. Hepatol. 28 (Suppl. 1), 125–131 (2013).
Zhang, K. et al. Hepatic suppression of Foxo1 and Foxo3 causes hypoglycemia and hyperlipidemia in mice. Endocrinology 153, 631–646 (2012).
Tao, R. et al. Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J. Biol. Chem. 286, 14681–14690 (2011).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
Sajan, M. P. et al. Akt-dependent phosphorylation of hepatic FoxO1 is compartmentalized on a WD40/ProF scaffold and is selectively inhibited by aPKC in early phases of diet-induced obesity. Diabetes 63, 2690–2701 (2014).
Hall, J. A., Tabata, M., Rodgers, J. T. & Puigserver, P. USP7 attenuates hepatic gluconeogenesis through modulation of FoxO1 gene promoter occupancy. Mol. Endocrinol. 28, 912–924 (2014).
Kato, S., Ding, J., Pisck, E., Jhala, U. S. & Du, K. COP1 functions as a FoxO1 ubiquitin E3 ligase to regulate FoxO1-mediated gene expression. J. Biol. Chem. 283, 35464–35473 (2008).
Mihaylova, M. M. et al. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 145, 607–621 (2011).
Nagashima, T. et al. Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: improvement of fasting glycemia in diabetic db/db mice. Mol. Pharmacol. 78, 961–970 (2010).
Tanaka, H. et al. Effects of the novel Foxo1 inhibitor AS1708727 on plasma glucose and triglyceride levels in diabetic db/db mice. Eur. J. Pharmacol. 645, 185–191 (2010).
Shaywitz, A. J. & Greenberg, M. E. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 68, 821–861 (1999).
Gonzalez, G. A. & Montminy, M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59, 675–680 (1989).
Chrivia, J. C. et al. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 365, 855–859 (1993).
Koo, S. H. et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 (2005).
Herzig, S. et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413, 179–183 (2001). The authors of this study use Creb -knockout mice and mice expressing a dominant-negative CREB inhibitor to show that CREB increases hepatic gluconeogenesis and blood glucose levels through direct activation of PGC1α expression.
Herzig, S. et al. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-γ. Nature 426, 190–193 (2003).
Lee, J. M. et al. AMPK-dependent repression of hepatic gluconeogenesis via disruption of CREB. CRTC2 complex by orphan nuclear receptor small heterodimer partner. J. Biol. Chem. 285, 32182–32191 (2010).
Dentin, R. et al. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 449, 366–369 (2007). This study demonstrates that TORC2 has a role in diabetic glucose homeostasis, as TORC2 levels are increased in diabetes and insulin inhibits the gluconeogenic gene programme through phosphorylation and subsequent degradation of TORC2.
Oh, K. J. et al. TCF7L2 modulates glucose homeostasis by regulating CREB- and FoxO1-dependent transcriptional pathway in the liver. PLoS Genet. 8, e1002986 (2012).
Zhang, E. E. et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 16, 1152–1156 (2010).
Hammitzsch, A. et al. CBP30, a selective CBP/p300 bromodomain inhibitor, suppresses human Th17 responses. Proc. Natl Acad. Sci. USA 112, 10768–10773 (2015).
Best, J. L. et al. Identification of small-molecule antagonists that inhibit an activator: coactivator interaction. Proc. Natl Acad. Sci. USA 101, 17622–17627 (2004). An NMR-based screen is used in this work to identify small molecules that bind to CREB and disrupt its interaction with CBP, which decreases the cellular response to cAMP, showing that small molecules can inhibit cAMP signalling through interference of nuclear protein–protein interactions.
Wang, Y. et al. Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc. Natl Acad. Sci. USA 107, 3087–3092 (2010).
Hirota, T. et al. Identification of small molecule activators of cryptochrome. Science 337, 1094–1097 (2012).
Wang, N. D. et al. Impaired energy homeostasis in C/EBP α knockout mice. Science 269, 1108–1112 (1995). The authors use Cebpa -knockout mice to demonstrate that C/EBPα is needed for full activation of glycogen synthase and genes involved in gluconeogenesis, as well as lipid accumulation in hepatocytes, thus establishing C/EBPα as a regulator of hepatic energy metabolism.
Pedersen, T. A. et al. Distinct C/EBPα motifs regulate lipogenic and gluconeogenic gene expression in vivo. EMBO J. 26, 1081–1093 (2007).
Qiao, L., MacDougald, O. A. & Shao, J. CCAAT/enhancer-binding protein α mediates induction of hepatic phosphoenolpyruvate carboxykinase by p38 mitogen-activated protein kinase. J. Biol. Chem. 281, 24390–24397 (2006).
Arizmendi, C., Liu, S., Croniger, C., Poli, V. & Friedman, J. E. The transcription factor CCAAT/enhancer-binding protein beta regulates gluconeogenesis and phosphoenolpyruvate carboxykinase (GTP) gene transcription during diabetes. J. Biol. Chem. 274, 13033–13040 (1999).
Watanabe, N. & Osada, H. Small molecules that target phosphorylation dependent protein-protein interaction. Bioorg. Med. Chem. http://dx.doi.org/10.1016/j.bmc.2016.03.023 (2016).
Solt, L. A., Kojetin, D. J. & Burris, T. P. The REV-ERBs and RORs: molecular links between circadian rhythms and lipid homeostasis. Future Med. Chem. 3, 623–638 (2011).
Yin, L. et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 318, 1786–1789 (2007). This work reveals that the haem sensor and circadian clock component REV-ERBα decreases glucose output and gluconeogenic gene expression in liver cells.
Liu, C., Li, S., Liu, T., Borjigin, J. & Lin, J. D. Transcriptional coactivator PGC-1α integrates the mammalian clock and energy metabolism. Nature 447, 477–481 (2007).
Chopra, A. R. et al. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science 322, 1395–1399 (2008).
Kadiri, S. et al. The nuclear retinoid-related orphan receptor-α regulates adipose tissue glyceroneogenesis in addition to hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 309, E105–E114 (2015).
Kojetin, D. J. & Burris, T. P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 13, 197–216 (2014).
Grant, D. et al. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbα. ACS Chem. Biol. 5, 925–932 (2010).
Solt, L. A. et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485, 62–68 (2012).
Kumar, N. et al. Identification of SR3335 (ML-176): a synthetic RORα selective inverse agonist. ACS Chem. Biol. 6, 218–222 (2011).
Xu, J., Wu, R. C. & O'Malley, B. W. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat. Rev. Cancer 9, 615–630 (2009).
Louet, J. F. et al. The coactivator SRC-1 is an essential coordinator of hepatic glucose production. Cell. Metab. 12, 606–618 (2010). The authors find that SRC1 is induced by fasting and increases hepatic glucose production through activation of C/EBPα, which transactivates pyruvate carboxylase.
Wang, Y. et al. Small molecule inhibition of the steroid receptor coactivators, SRC-3 and SRC-1. Mol. Endocrinol. 25, 2041–2053 (2011).
Rousset, S. et al. The biology of mitochondrial uncoupling proteins. Diabetes 53 (Suppl. 1), S130–S135 (2004).
Grundlingh, J., Dargan, P. I., El-Zanfaly, M. & Wood, D. M. 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. J. Med. Toxicol. 7, 205–212 (2011).
Perry, R. J. et al. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell. Metab. 18, 740–748 (2013).
Perry, R. J., Zhang, D., Zhang, X. M., Boyer, J. L. & Shulman, G. I. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science 347, 1253–1256 (2015). This study demonstrates that a controlled-release mitochondrial protonophore induces mild hepatic mitochondrial uncoupling and decreases insulin resistance and diabetes in rats without causing systemic toxicity.
Consoli, A., Nurjhan, N., Capani, F. & Gerich, J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes 38, 550–557 (1989).
Moore, M. C., Coate, K. C., Winnick, J. J., An, Z. & Cherrington, A. D. Regulation of hepatic glucose uptake and storage in vivo. Adv. Nutr. 3, 286–294 (2012).
Lin, H. V. & Accili, D. Hormonal regulation of hepatic glucose production in health and disease. Cell. Metab. 14, 9–19 (2011). This review provides an overview of the regulatory mechanisms of hepatic glucose production, as well as a discussion of the controversies surrounding this topic.
Rizza, R. A. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes 59, 2697–2707 (2010).
Bandsma, R. H. et al. Acute inhibition of glucose-6-phosphate translocator activity leads to increased de novo lipogenesis and development of hepatic steatosis without affecting VLDL production in rats. Diabetes 50, 2591–2597 (2001).
Lei, K. J. et al. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse. Nat. Genet. 13, 203–209 (1996).
Clar, J. et al. Targeted deletion of kidney glucose-6 phosphatase leads to nephropathy. Kidney Int. 86, 747–756 (2014).
van Schaftingen, E. & Gerin, I. The glucose-6-phosphatase system. Biochem. J. 362, 513–532 (2002).
Wulffele, M. G., Kooy, A., de Zeeuw, D., Stehouwer, C. D. & Gansevoort, R. T. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: a systematic review. J. Internal Med. 256, 1–14 (2004).
Befroy, D. E. et al. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nat. Med. 20, 98–102 (2014).
Inzucchi, S. E., Lipska, K. J., Mayo, H., Bailey, C. J. & McGuire, D. K. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 312, 2668–2675 (2014).
Wiernsperger, N. F. & Bailey, C. J. The antihyperglycaemic effect of metformin: therapeutic and cellular mechanisms. Drugs 58 (Suppl. 1), 31–39; discussion 75–82 (1999).
El-Mir, M. Y. et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 (2000).
Owen, M. R., Doran, E. & Halestrap, A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 (2000).
Foretz, M. et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 120, 2355–2369 (2010).
Shaw, R. J. et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005).
Miller, R. A. et al. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 494, 256–260 (2013).
Madiraju, A. K. et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510, 542–546 (2014).
Fullerton, M. D. et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 19, 1649–1654 (2013).
Dornhorst, A. Insulinotropic meglitinide analogues. Lancet 358, 1709–1716 (2001).
Brown, D. X. & Evans, M. Choosing between GLP-1 receptor agonists and DPP-4 inhibitors: a pharmacological perspective. J. Nutr. Metab. 2012, 381713 (2012).
Zinman, B. et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 373, 2117–2128 (2015).
Bailey, T. Options for combination therapy in type 2 diabetes: comparison of the ADA/EASD position statement and AACE/ACE algorithm. Am. J. Med. 126, S10–S20 (2013).
Rojas, L. B. & Gomes, M. B. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol. Metab. Synd. 5, 6 (2013).
Inzucchi, S. E. et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38, 140–149 (2015).
van Poelje, P. D., Potter, S. C. & Erion, M. D. Fructose-1, 6-bisphosphatase inhibitors for reducing excessive endogenous glucose production in type 2 diabetes. Handb. Exp. Pharmacol. 203, 279–301 (2011).
Bantubungi, K. et al. Cdkn2a/p16Ink4a regulates fasting-induced hepatic gluconeogenesis through the PKA–CREB–PGC1α pathway. Diabetes 63, 3199–3209 (2014).
Acknowledgements
A.K.R. received funding from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) at the US National Institutes of Health (NIH) (F32 DK102293-01). K.S. was funded by the American Heart Association (15POST22880002). C.D.J.T. received funding from the American Diabetes Association (1-16-PDF-111). P.P. was funded by the NIH NIDDK (R01 DK069966, DK081418, DK089883) and the American Diabetes Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Type 2 diabetes
-
A chronic disease of aberrant glucose homeostasis that is characterized by elevated blood glucose levels and insulin resistance.
- Sulfonylureas
-
Type 2 diabetes drugs that stimulate insulin secretion from the pancreas through inhibition of KATP channels in β-cells.
- Thiazolidinediones
-
Insulin-sensitizing type 2 diabetes drugs that act as peroxisome proliferator-activated receptor-γ (PPARγ) agonists (also known as glitazones).
- Gluconeogenesis
-
A metabolic pathway utilizing carbon substrates to generate glucose.
- Hyperinsulinaemia
-
Excess insulin levels in the blood, often caused by insulin resistance.
- Hypoglycaemia
-
Suppressed blood glucose below normal levels.
- Glycogenolysis
-
A metabolic pathway that breaks down glycogen into glucose.
- Hyperglycaemia
-
Elevated blood glucose above normal levels.
- Hepatic steatosis
-
Fatty liver disease.
- Lactic acidosis
-
Increased acidity in the body due to a build-up of lactate; a potential complication associated with inhibition of hepatic gluconeogenesis.
- Insulin secretagogues
-
Agents that increase insulin release from the pancreas.
Rights and permissions
About this article
Cite this article
Rines, A., Sharabi, K., Tavares, C. et al. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat Rev Drug Discov 15, 786–804 (2016). https://doi.org/10.1038/nrd.2016.151
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2016.151
This article is cited by
-
Resveratrol attenuates high glucose-induced inflammation and improves glucose metabolism in HepG2 cells
Scientific Reports (2024)
-
Structural Analysis and Novel Mechanism of Enteromorpha prolifera Sulfated Polysaccharide in Preventing Type 2 Diabetes Mellitus
Plant Foods for Human Nutrition (2024)
-
Exploration of the anti-diabetic potential of hydro-ethanolic leaf extract of Koenigia polystachya L.: an edible wild plant from Northeastern India
Laboratory Animal Research (2023)
-
Characteristics of glucose and lipid metabolism and the interaction between gut microbiota and colonic mucosal immunity in pigs during cold exposure
Journal of Animal Science and Biotechnology (2023)
-
Regulation of biological processes by intrinsically chiral engineered materials
Nature Reviews Materials (2023)