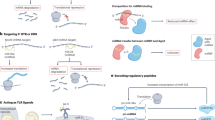
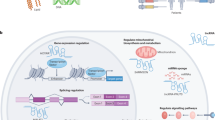
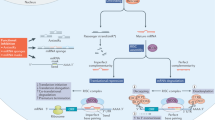
Key Points
-
Non-coding RNAs (ncRNAs) — which include microRNAs (miRNAs), repetitive RNAs, intronic RNAs, and long ncRNAs (lncRNAs) — are a diverse group of biomolecules with broad potential to control gene expression.
-
Compounds that target ncRNAs have the potential to control expression of disease-related genes
-
Development of compounds to target ncRNAs can benefit from understanding the lessons learnt from decades of research using antisense oligonucleotides (ASOs) and duplex RNAs to control expression of mRNA.
-
ASOs that affect splicing or that are complementary to miRNAs are already being tested in multiple clinical trials in a variety of diseases, including cancer and muscular dystrophy
-
lncRNAs can affect transcription or splicing and are emerging as a promising class of novel drug targets.
Abstract
Most of the human genome encodes RNAs that do not code for proteins. These non-coding RNAs (ncRNAs) may affect normal gene expression and disease progression, making them a new class of targets for drug discovery. Because their mechanisms of action are often novel, developing drugs to target ncRNAs will involve equally novel challenges. However, many potential problems may already have been solved during the development of technologies to target mRNA. Here, we discuss the growing field of ncRNA — including microRNA, intronic RNA, repetitive RNA and long non-coding RNA — and assess the potential and challenges in their therapeutic exploitation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Morris, K. V. & Mattick, J. S. The rise of regulatory RNA. Nat. Rev. Genet. 15, 423–437 (2014).
Ling, H., Fabbri, M. & Calin, G. A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug. Discov. 12, 847–865 (2013).
St. Laurent, G., Wahlestedt, C. & Kapranov, P. The landscape of long noncoding RNA classification. Trends Genet. 31, 239–251 (2015).
Ebbersen, K. K., Kjems, J. & Hansen, T. B. Circular RNAs: identification, biogenesis and function. Biochim. Biophys. Acta 1859, 163–168 (2016).
Sato-Kuwabara, Y., Melo, S. A., Soares, F. A. & Calin, G. A. The fusion of two worlds: noncoding RNAs and extracellular RNAs — diagnostic and therapeutic implications. Int. J. Oncol. 46, 17–27 (2015).
van Rooij, E. & Kauppinen, S. Development of microRNA therapeutics is coming of age. EMBO Mol. Med. 6, 851–864 (2014).
Zamencnik, P. C. & Stephenson, M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligonucleotide. Proc. Natl Acad. Sci. USA 75, 280–284 (1978).
Sharma, V. K. & Watts, J. K. Oligonucleotide therapeutics: chemistry, delivery and clinical progress. Future Med. Chem. 7, 2221–2242 (2015).
Schmidt, M. F. Drug target miRNAs: chances and challenges. Trends Biotechnol. 32, 578–585 (2014).
Deleavey, G. F. & Damha, M. J. Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 19, 937–954 (2012).
Ozcan, G., Ozpolat, B., Coleman, R. L., Sood, A. K. & Lopez-Berestein, G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 87, 108–119 (2015).
Sah, D. W. Y. & Aronin, N. Oligonucleotide therapeutic approaches for Huntington disease J. Clin. Invest. 121, 500–507 (2011).
Doak, B. C., Over, B., Giordanetto, F. & Kihlberg, J. Oral druggable space beyond the rule of 5: insights from drugs and clinical candidates. Chem. Biol. 21, 1115–1142 (2014).
Bennett, C. F. & Swayze, E. E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 50, 259–293 (2010).
Abramova, T. Frontiers and approaches to chemical synthesis of oligodeoxyribonucleotides. Molecules 18, 1063–1075 (2013).
Juliano, R. L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. http://dx.doi.org/10.1093/nar/gkw236 (2016).
Geary, R. S., Henry, S. P. & Grillone, L. R. Fomivirsen: clinical pharmacology and potential drug interactions. Clin. Pharmacokinet. 41, 255–260 (2002).
Rader, D. J. & Kastelein, J. J. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation 129, 1022–1032 (2014). A demonstration that ASOs can have profound effects on gene expression in humans.
McClorey, G. & Wood, M. J. An overview of clinical application of antisense oligonucleotides for RNA-targeting therapies. Curr. Opin. Pharmacol. 24, 52–58 (2015).
Childs-Disney, J. L. & Disney, M. D. Approaches to validate and manipulate RNA targets with small molecules in cells. Annu. Rev. Pharmacol. Toxicol. 56, 123–140 (2016).
Watts, J. K. & Corey, D. R. Silencing disease genes in the laboratory and the clinic. J. Pathol. 226, 365–379 (2012).
Wilson, R. C. & Doudna, J. A. Molecular mechanisms of RNA interference. Annu. Rev. Biophys. 42, 217–239 (2013).
Crooke, S. T. Proof of mechanism of antisense drugs. Antisense Nucleic Acid. Drug Dev. 6, 145–147 (1996).
Stein, C. A. Keeping the biotechnology of antisense in context. Nat. Biotechnol. 17, 209 (1999).
Crooke, S. T. Evaluating the mechanism of action of antiproliferative antisense drugs. Antisense Nucleic Acid. Drug Dev. 10, 123–126 (2000).
[No authors listed.] Whither RNAi? Nat. Cell. Biol. 5, 489–490 (2003). Along with references 23–25, this work provides common sense guidelines for interpreting experiments and designing controls.
Sledz, C. A. & Williams, B. R. RNA interference and double-stranded-RNA-activated pathways. Biochem. Soc. Trans. 32, 952–956 (2004).
Svoboda, P. Off-targeting and other non-specific effects of RNAi experiments in mammalian cells. Curr. Opin. Mol. Ther. 9, 248–257 (2007).
Juliano, R. L. & Carver, K. Cellular uptake and intracellular trafficking of oligonucleotides. Adv. Drug. Deliv. Rev. 87, 35–45 (2015).
Myers, K. J. & Dean, N. M. Sensible use of antisense: how to use oligonucleotides as research tools. Trends Pharmcol. Sci. 21, 19–23 (2000).
Stein, C. A. The experimental use of antisense oligonucleotides: a guide for the perplexed. J. Clin. Invest. 108, 641–644 (2001).
Krieg, A. M. CpG still rocks. Update on an accidental drug. Nucleic Acid. Ther. 22, 77–89 (2012).
Chen, Z., Monia, B. P. & Corey, D. R. Telomerase inhibition, telomere shortening, and decreased cell proliferation by cell permeable 2′-O-methoxyethyl oligonucleotides. J. Med. Chem. 45, 5423–5425 (2002).
Stein, C. A. et al. Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 38, e3 (2010). Reported application of 'gymnotic' oligonucleotide delivery, providing an important tool for experimenters using ASOs.
Jonas, S. & Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 16, 421–433 (2015).
Hammond, S. M. An overview of microRNAs. Adv. Drug Deliv. Rev. 87, 3–14 (2015).
Li, Z. & Rana, T. M. Therapeutic targeting of microRNAs: current status and future challenges. Nat. Rev. Drug Discov. 13, 622–638 (2014).
Kaboli, P. J., Rahmat, A., Ismail, P. & Ling, K.-H. MicroRNA-based therapy and breast cancer: a comprehensive review of novel therapeutic strategies from diagnosis to treatment. Pharm. Res. 97, 104–121 (2015).
Agostini, M. & Knight, R. A. miR-34: from bench to bedside. Oncotarget 5, 872–881 (2014).
Thakral, S. & Ghoshal, K. miR-122 is a unique molecule with great potential in the diagnosis, prognosis of liver disease and therapy both as miRNA mimic and antimir. Curr. Gene Ther. 15, 142–150 (2015).
Gomez, I. G. et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J. Clin. Invest. 125, 141–156 (2015).
Venter, J. C. et al. The sequence of the human genome. Science 291, 1304–1351 (2001).
Aartsma-Rus, A. & van Ommen, G.-J. B. Antisense-mediated exon skipping: a versatile tool with therapeutic and research applications. RNA 13, 1609–1624 (2007).
Järver, P., O'Donovan, L. & Gait, M. J. A chemical view of oligonucleotides for exon skipping and related drug applications. Nucleic Acid. Ther. 24, 37–47 (2014).
Rigo, F., Seth, P. P. & Bennett, C. F. Antisense oligonucleotide-based therapies for diseases caused by pre-mRNA processing defects. Adv. Exp. Med. Biol. 825, 303–352 (2014).
Dominski, Z. & Kole, R. Restoration of correct splicing in thalassemic pre-mRNA by antisense oligonucleotides. Proc. Natl Acad. Sci. USA 90, 8673–8677 (1993). Classic work introducing the concept of ASO-mediated regulation of splicing.
Rigo, F. et al. Synthetic oligonucleotides recruit ILF2/3 to RNA transcripts to modulate splicing. Nat. Chem. Biol. 8, 555–561 (2012).
Sierakowska, H., Sambade, M. J., Agrawal, S. & Kole, R. Repair of thalassemic human β-globin mRNA in mammalian cells by antisense oligonucleotides. Proc. Natl Acad. Sci. USA 93, 12840–12844 (1996).
Mann, C. J. et al. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc. Natl Acad. Sci. USA 98, 42–47 (2001).
van Deutekom, J. C. et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N. Engl. J. Med. 357, 2677–2686 (2007).
Goemans, N. M. et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N. Engl. J. Med. 364, 1513–1522 (2011).
Voit, T. et al. Safety and efficacy of drisapersen for the treatment of Duchenne muscular dystrophy (DEMAND II): an exploratory randomised, placebo-controlled Phase 2 study. Lancet Neurol. 13, 987–996 (2014).
Lentz, J. J. et al. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nat. Med. 19, 345–350 (2013).
Passini, M. A. et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med. 3, 72ra18 (2011).
Kole, R. & Krieg, A. M. Exon skipping therapy for Duchenne muscular dystrophy. Adv. Drug. Deliv. Rev. 87, 104–107 (2015).
Mendell, J. R. et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann. Neurol. 79, 257–271 (2016).
Chiriboga, C. A. et al. Results from a phase 1 study of nusinersen (ISIS-SMNRx) in children with spinal muscular atrophy. Neurology 86, 890–897 (2016). Compelling early stage clinical results of an ASO designed to change splicing and upregulate functional SMN protein.
[No authors listed.] FDA briefing document: Peripheral and Central Nervous System Drugs Advisory Committee Meeting, November 24, 2015, NDA 206031. US Food and Drug Administration http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/peripheralandcentralnervoussystemdrugsadvisorycommittee/ucm473737.pdf (2014)
Lu, Q. L., Cirak, S. & Partridge, T. What can we learn from clinical trials of exon skipping for DMD? Mol. Ther. Nucleic Acids 3, e152 (2014).
Kimberling, W. J. et al. Frequency of Usher syndrome in two pediatric populations: implications for genetic screening of deaf and hard of hearing children. Genet. Med. 12, 512–526 (2010).
Ebermann, I. et al. Deafblindness in French Canadians from Quebec: a predominant founder population in USH1C gene provides the first genetic link with the Acadian population. Genome. Biol. 8, R47 (2007).
Faravelli, I., Nizzardo, M., Comi, G. P. & Corti, S. Spinal muscular atrophy — recent therapeutic advances for an old challenge. Nat. Rev. Neurol. 11, 351–359 (2015).
Ionis Pharmaceuticals, Inc. Ionis Pharmaceuticals Reports Data Update from Nusinersen Phase 2 Study in Infants with Spinal Muscular Atrophy and Reviews Neurological Disease Franchise. Ionis Pharmaceuticals Press Release http://ir.ionispharma.com/phoenix.zhtml?c=222170&p=irol-newsArticle&ID=2158750 (2016).
Schmidt, M. H. M. & Pearson, C. E. Disease-associate repeat instability and mismatch repair. DNA Repair 38, 117–126 (2016).
Nelson, D. L., Orr, H. T. & Warren, S. T. The unstable repeats — three evolving faces of neurological disease. Neuron 77, 825–843 (2013).
La Spada, A. R. & Taylor, J. P. Repeat expansion disease:progress and puzzles in disease pathogenesis. Nat. Rev. Genet. 11, 247–258 (2010).
Kay, C., Skotte, N. H., Southwell, A. L. & Hayden, M. R. Personalized gene silencing therapeutics for Huntington disease. Clin. Genet. 86, 29–36 (2014).
Fiszer, A. & Krzyzosiak, W. J. Oligonucleotide-based strategies to combat polyglutamine diseases. Nucleic Acids Res. 42, 6787–6810 (2014).
Mankodi, A. et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 289, 1769–1772 (2000).
Kuyumcu-Martinez, N. M., Wang, G.-S. & Cooper, T. A. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol. Cell 28, 68–78 (2007).
Mulders, S. A. et al. Triplet-repeat oligonucleotide-mediated reversal of RNA toxicity in myotonic dystrophy. Proc. Natl Acad. Sci. USA 106, 13915–13920 (2009).
Wheeler, T. M. et al. Reversal of RNA dominance by displacement of protein sequestered on triplet repeat RNA. Science 325, 336–339 (2009). This study demonstrated that ASOs can target trinucleotide repeats and detailed the investigation of the mechanism.
Pandey, S. K. et al. Identification and characterization of modified antisense oligonucleotides targeting DMPK in mice and nonhuman primates for the treatment of myotonic dystrophy type 1. J. Pharmacol. Exp. Ther. 355, 329–340 (2015).
Wheeler, T. M. et al. Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature 488, 111–115 (2012).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02312011 (2014).
Sandi, C., Sandi, M., Anjomani Virmouni, S., Al-Mahdawi, S. & Pook, M. A. Epigenetic-based therapies for Friedreich ataxia. Front. Genet. 5, 165 (2014).
Groh, M., Lufino, M. M. P., Wade-Martins, R. & Gromak, N. R-loops associated with triplet repeat expansions promote gene silencing in Friedreich ataxia and fragile X syndrome. PLoS Genet. 10, e1004318 (2014). This paper outlines the mechanism for inhibition of transcription in triplet-repeat disease genes, FXN and Fragile X mental retardation 1 ( FMR1 ), by R-loop formation.
Li, L., Matsui, M. & Corey, D. R. Activating frataxin expression by repeat-targeted nucleic acids. Nat. Commun. 7, 10606 (2016). An example of how targeting an intron with ASOs or dsRNAs can derepress a gene by blocking R-loop formation.
Yelin, R. et al. Widespread occurrence of antisense transcription in the human genome. Nat. Biotechnol. 21, 379–386 (2003).
Katayama, S. et al. Antisense transcription in the mammalian transcriptome. Science 309, 1564–1566 (2005).
Carninci, P. et al. The transcriptional landscape of the mammalian genome. Science 309, 1559–1563 (2005).
Kapranov, P. et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 316, 1484–1488 (2007).
Djebali, S. et al. Landscape of transcription in human cells. Nature 489, 101–108 (2012).
Bergmann, J. H. & Spector, D. L. Long non-coding RNAs: modulators of nuclear structure and function. Curr. Opin. Cell Biol. 26, 10–18 (2014).
Guttman, M. et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458, 223–227 (2009).
St. Laurent, G. et al. Functional annotation of vlinc class of non-coding RNAs using systems biology approach. Nucleic Acids Res. 44, 3233–3252 (2016).
van Bakel, H., Nislow, C., Blencowe, B. J. & Hughes, T. R. Most “dark matter” transcripts are associated with known genes. PLoS Biol. 8, e1000371 (2010).
Clark, M. B. et al. The reality of pervasive transcription. PLoS Biol. 9, e1000625 (2011).
van Bakel, H., Nislow, C., Blencowe, B. J. & Hughes, T. R. Response to “The Reality of Pervasive Transcription”. PLoS Biol. 9, e1001102 (2011). Together with references 87 and 88, this work illustrates the spirited debate over the broader importance of lncRNAs.
Bustin, S. A. Why the need for qPCR publication guidelines? — The case for MIQE. Methods 50, 217–226 (2010).
Dodd, D. W., Gagnon, K. T. & Corey, D. R. Digital quantitation of potential therapeutic target RNAs. Nucleic Acids Ther. 23, 188–194 (2013). An example of how RNA copy numbers can be quantified.
Gagnon, K. T., Li, L., Chu, Y. Janowski, B. A. & Corey, D. R. RNAi factors are present and active in human cell nuclei. Cell Rep. 6, 211–221 (2014).
Gagnon, K. T., Li, L., Janowski, B. A. & Corey, D. R. Analysis of nuclear RNA interference in human cells by subcellular fractionation and Argonaute loading. Nat. Protoc. 9, 2045–2060 (2014).
Lennox, K. A. & Behlke, M. A. Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides. Nucleic Acids Res. 44, 863–877 (2016). This study makes the important observation that ASOs function best in nuclei, whereas duplex RNAs function best in the cytoplasm.
Wahlestedt, C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat. Rev. Drug Discov. 12, 433–446 (2013).
Froberg, J. E., Yang, L. & Lee, J. T. Guided by RNAs: X-inactivation as a model for lncRNA function. J. Mol. Biol. 425, 3698–3706 (2013).
Davidovich, C. & Cech, T. R. The recruitment of chromatin modifiers by long noncoding RNAs: lessons from PRC2. RNA 21, 2007–2022 (2015).
Simon, J. A. & Kingston, R. E. Occupying chromatin: polycomb mechanisms for getting to genomic targets, stopping transcriptional traffic, and staying put. Mol. Cell 49, 808–824 (2013).
Plath, K. et al. Role of histone H3 lysine 27 methylation in X inactivation. Science 300, 131–135 (2003).
Zhao, J., Sun, B. K., Erwin, J. A., Song, J.-J. & Lee, J. T. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322, 750–756 (2008).
Khalil, A. M. et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl Acad. Sci. USA 106, 11667–11672 (2009).
Sarma, K., Levasseur, P., Aristarkhov, A. & Lee, J. T. Locked nucleic acids (LNAs) reveal sequence requirements and kinetics of Xist RNA localization to the X chromosome. Proc. Natl Acad. Sci. USA 107, 22196–22201 (2010).
Davidovich, C., Zheng, L., Goodrich, K. J. & Cech, T. R. Promiscuous RNA binding by Polycomb repressive complex 2. Nat. Struct. Mol. Biol. 20, 1250–1257 (2013).
Wang, X., Schwartz, J. C. & Cech, T. R. Nucleic acid-binding specificity of human FUS protein. Nucleic Acids Res. 43, 7535–7543 (2015).
Davidovich, C. et al. Towards a consensus on the binding specificity and promiscuity of PRC2 for RNA. Mol. Cell 57, 552–558 (2015). A balanced and nuanced investigation from two groups with different viewpoints of the role of PRC2 and its interactions with RNA.
Kalantari, R., Chiang, C.-M. & Corey, D. R. Regulation of mammalian transcription and splicing by nuclear RNAi. Nucleic Acids Res. 44, 524–537 (2015).
Roberts, T. C. The microRNA biology of the mammalian nucleus. Mol. Ther. Nucleic Acids 3, e188 (2014).
Matsui, M. et al. Promoter RNA links transcriptional regulation of inflammatory pathway genes. Nucleic Acids Res. 41, 10086–10109 (2013). An in-depth study of one mechanism for controlling transcription with miRNAs and synthetic duplex RNAs.
Liu, J., Hu, J. & Corey, D. R. Expanding the action of duplex RNAs into the nucleus: redirecting alternative splicing. Nucleic Acids Res. 40, 1240–1250 (2012).
Modarresi, F. et al. Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat. Biotechnol. 30, 453–459 (2012).
Ji, P. et al. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 22, 8031–8041 (2003).
Tripathi, V. et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 39, 925–938 (2010).
Zhang, B. et al. The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis regulatory role in the adult. Cell Rep. 2, 111–123 (2012).
Eissmann, M. et al. Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. RNA Biol. 9, 1076–1087 (2012).
Nakagawa, S. et al. MALAT1 is not an essential component of nuclear speckles in mice. RNA 18, 1487–1499 (2012).
Arun, G. et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 30, 34–51 (2016).
Gutschner, T. et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 73, 1180–1189 (2013).
Fan, Y. et al. TGF-β-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin. Cancer Res. 20, 1531–1541 (2014).
Engreitz, J. M. et al. RNA–RNA interactions enable specific targeting of noncoding RNAs to nascent pre-mRNAs and chromatin sites. Cell 159, 188–199 (2014).
West, J. A. et al. The noncoding RNAs NEAT1 and MALAT1 bind active chromatin sites. Mol. Cell 55, 791–802 (2014).
Yoshimoto, R., Mayeda, A., Yoshida, M. & Nakagawa, S. MALAT1 and noncoding RNA in cancer. Biochim. Biophys. Acta 1859, 192–199 (2016).
Bird, L. M. Angelman syndrome: review of clinical and molecular aspects. Appl. Clin. Genet. 7, 93–104 (2014).
Rougeulle, C., Cardoso, C., Fontés, M., Colleaux, L. & Lalande, M. An imprinted antisense RNA overlaps UBE3A and a scond maternally expressed transcript. Nat. Genet. 19, 15–16 (1998).
Chamberlain, S. J. & Brannan, C. I. The Prader-Willi syndrome imprinting center activates the paternally expressed murine Ube3a antisense transcript but represses paternal Ube3a. Genomics 73, 316–322 (2001).
Meng, L., Person, R. E. & Beaudet, A. L. Ube3a-ATS is an atypical RNA polymerase II transcript that represses the paternal expression of Ube3a. Hum. Mol. Genet. 21, 3001–3012 (2012).
Meng, L. et al. Towards a therapy for Angelman syndrome by targeting a long noncoding RNA. Nature 518, 409–412 (2015).
Lee, S. et al. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell 164, 69–80 (2016). This study provides an unusually detailed mechanism of action for a well-expressed lncRNA.
Leucci, E. et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature 531, 518–522 (2016).
Fogal, V. et al. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol. Cell. Biol. 30, 1303–1318 (2010).
Begley, C. G. & Ellis, L. M. Drug development: raise standards for preclinical cancer research. Nature 483, 531–533 (2012). This paper points out the low rate of reproducibility for many published results.
Freedman, L. P. & Inglese, J. The increasing urgency for standards in basic biologic research. Cancer Res. 74, 4024–4029 (2014).
[No authors listed.] Oblimersen: Augmersosen, BCL-2 antisense oligonucleotide — Genta, G 3139, GC 3139, oblimersen sodium. Drugs R. D. 8, 321–334 (2007).
Kim, R., Emi, M., Tababe, K. & Toge, T. Therapeutic potential of antisense Bcl-2 as a chemosensitizer for cancer therapy. Cancer 101, 2491–2501 (2004).
Bedikian, A. Y. et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J. Clin. Oncol. 24, 4738–4745 (2006).
Bedikian, A. Y. et al. Dacarbazine with or without oblimersen (a Bcl-2 antisense oligonucleotide) in chemotherapy-naive patients with advanced melanoma and low-normal serum lactate dehydrogenase: 'the AGENDA trial'. Melanoma Res. 24, 237–243 (2014).
Lai, J. C. et al. G3139 (oblimersen) may inhibit prostate cancer cell growth in a partially bis-CpG-dependent non-antisense manner. Mol. Cancer Ther. 2, 1031–1043 (2003).
Benimetskaya, L. et al. Relative Bcl-2 independence of drug-induced cytotoxicity and resistance in 518A2 melanoma cells. Clin. Cancer Res. 10, 8371–8379 (2004).
Anderson, E. M. et al. Gene profiling study of G3139- and Bcl-2 targeting siRNAs identifies a unique G3139 molecular signature. Cancer Gene Ther. 13, 406–414 (2006).
Winkler, J., Stessl, M., Amartey, J. & Noe, C. R. Off-target effects related to the phosphorothioate modifications of nucleic acids. ChemMedChem. 5, 1344–1352 (2010).
Gjertsen, B. T., Bredholt, T., Anensen, N. & Vintemyr, O. K. Bcl-2 antisense in the treatment of human malignancies: a delusion in targeted therapy. Curr. Pharm. Biotechnol. 8, 373–381 (2007).
Westphal, S. P. Behind the mask. New Scientist 179, 32–35 (2003).
Beg, M. S. et al. Safety, tolerability, and clinical activity of MRX34, the first-in-class liposomal miR-34 mimic, in patients with advanced solid tumors. Mol. Cancer. Ther. 14 (12 Suppl. 2) C43 (2015).
Regulus Therapeutics Inc. All HCV patients treated with a single SC administration of 4 mg/kg of RG-101 responded with mean viral load reduction of 4.8 log10 at day 29 and 9/14 patients are below the limit of quantification at day 57. Regulus Therapeutics press release, http://ir.regulusrx.com/releasedetail.cfm?ReleaseID=895314 (2015).
Regulus Therapeutics Inc. RG-101 interim analysis shows 97% response at 8 week follow-up. Regulus Therapeutics press release, http://ir.regulusrx.com/releasedetail.cfm?ReleaseID=955249 (2016).
Regulus Therapeutics Inc. Regulus to present additional preclinical data supporting RG-012 as a novel microRNA therapeutic in development for Alport Syndrome at ASN's Kidney Week 2015. Regulus Therapeutics press release, http://ir.regulusrx.com/releasedetail.cfm?ReleaseID=935181 (2015).
Regulus Therapeutics Inc. RG-125(AZD4076), a microRNA therapeutic targeting microRNA-103/107 being developed for the treatment of NASH in patients with type 2 diabetes/pre-diabetes, enters Phase I clinical development. Regulus Therapeutics press release, http://ir.regulusrx.com/releasedetail.cfm?ReleaseID=947579 (2015).
Janssen, H. L. A. et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 368, 1685–1694 (2013).
Gebert, L. F. et al. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 42, 609–621 (2014).
Ottosen, S. et al. In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrob. Agents Chemother. 59, 599–608 (2015).
van der Ree, M. H. et al. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment. Pharmacol. Ther. 43, 102–113 (2016).
BioMarin Pharmaceutical Inc. BioMarin announces withdrawal of market authorization application for Kyndrisa™ (drisapersen) in Europe. BioMarin Pharmaceutical press release, http://investors.bmrn.com/releasedetail.cfm?ReleaseID=973536 (2016).
Haché, M. et al. Intrathecal injections in children with spinal muscular atrophy: Nusinersen clinical trial experience. J. Child Neurol. 31, 899–906 (2016).
Acknowledgements
This work was supported by the National Institutes of Health (GM106151, GM73042 and GM118103) and the Robert Welch Foundation (I-1244). D.R.C. holds the Rusty Kelley Professorship in Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Antisense oligonucleotides
-
(ASOs). Synthetic single-stranded oligonucleotides that are designed to bind to complementary cellular RNA sequences by Watson–Crick base pairing.
- Droplet digital PCR
-
(ddPCR). A PCR technology that uses nanolitre-sized oil–water emulsion droplets as PCR reaction vessels and quantifies concentrations of target DNA templates based on counting the number of PCR-positive droplets using a flow cytometer.
- Long non-coding RNAs
-
(lncRNAs). Relatively long (>200 nucleotides) non-coding RNA transcripts.
- MicroRNAs
-
(miRNAs). Small (~22 nucleotides) non-coding transcripts that are generally thought to silence gene translation through RNA interference.
- Non-coding RNAs
-
(ncRNAs). RNA transcripts that do not code for protein.
- Off-target effect
-
Phenotypic effects in a cell or animal that occur upon addition of a synthetic compound and that are not caused by interactions with the intended cellular target.
Rights and permissions
About this article
Cite this article
Matsui, M., Corey, D. Non-coding RNAs as drug targets. Nat Rev Drug Discov 16, 167–179 (2017). https://doi.org/10.1038/nrd.2016.117
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2016.117
This article is cited by
-
Identification of functional genes in liver fibrosis based on bioinformatics analysis of a lncRNA-mediated ceRNA network
BMC Medical Genomics (2024)
-
Long noncoding RNA UNC5B-AS1 suppresses cell proliferation by sponging miR-24-3p in glioblastoma multiforme
BMC Medical Genomics (2024)
-
Epiallelic variation of non-coding RNA genes and their phenotypic consequences
Nature Communications (2024)
-
Non-coding RNAs in disease: from mechanisms to therapeutics
Nature Reviews Genetics (2024)
-
RNAi-based drug design: considerations and future directions
Nature Reviews Drug Discovery (2024)