Key Points
-
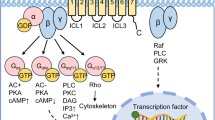
G protein-coupled receptors (GPCRs) can affect insulin action, insulin secretion and β-cell expansion, and certain GPCRs have emerged as potential drug targets for the development of anti-diabetic therapeutics.
-
Decreased insulin sensitivity is a major metabolic defect in the great majority of individualswith type 2 diabetes (T2D), and one of the key mechanisms underlying insulin resistance is chronic tissue inflammation.
-
Leukotriene B4 (LTB4) receptor 1 (LTB4R1) inhibitors may be anti-diabetic insulin sensitizers, as they inhibit inflammation and directly block the capacity of LTB4 to impair cellular insulin signalling in hepatocytes and myocytes.
-
The long-chain fatty acid receptors, free fatty acid receptor 1 (FFAR1, also known as GPR40) and FFAR4 (also known as GPR120), mediate beneficial effects by promoting glucose-induced insulin secretion or by inhibiting inflammatory signalling in immune cells, respectively.
-
The stimulation of β-cell glucose-dependent insulinotropic receptor (GPR119) leads directly to an increase in insulin secretion, and agonism of GPR119 on enteroendocrine cells promotes both glucagon-like peptide 1 (GLP1) and gastric inhibitory polypeptide (GIP) release. Therefore, GPR119 could be an important anti-diabetic drug target to promote insulin secretion.
-
Administration of exogenous CX3C-chemokine ligand 1 (CX3CL1, also known as fractalkine) to mice strikingly improves glucose tolerance and enhances β-cell insulin secretion. CX3CL1 and its receptor, CX3CR1, could be new targets used to positively influence β-cell function, and CX3CL1-based reagents might be future therapeutic approaches to anti-diabetes therapy.
Abstract
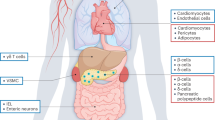
The prevalence of obesity and type 2 diabetes (T2D) is increasing worldwide, and these two metabolic disorders are closely linked. Lifestyle modification, including weight loss and exercise, are effective treatments for T2D, but, unfortunately, most patients are unsuccessful at maintaining durable weight reduction and recidivism is all too common. Therefore, safe and efficacious drugs are required for the successful treatment of T2D in a large proportion of patients. Targeting G protein-coupled receptors (GPCRs) in metabolic tissues — such as adipose tissue, liver, muscle, pancreatic islets, immune cells and the central nervous system — has emerged as a key target for current and future anti-diabetic compounds. This Opinion focuses on the potential of GPCRs as targets for the discovery of new drugs to successfully treat T2D.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
17 February 2016
In the original article, α-linolenic acid was incorrectly written. The error has been corrected in the print and online versions of the article.
References
Kahn, S. E., Hull, R. L. & Utzschneider, K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006).
Olefsky, J. M. & Courtney, C. H. in Textook of Endocrinology 5th edn (eds De Groot, J.& Jameson, J. L.) 1093–1117 (W. B. Saunders and Company, 2005).
Stumvoll, M., Goldstein, B. J. & van Haeften, T. W. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365, 1333–1346 (2005).
Barnes, A. S. The epidemic of obesity and diabetes: trends and treatments. Tex. Heart Inst. J. 38, 142–144 (2011).
Cerf, M. E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. (Lausanne) 4, 37 (2013).
Nolan, C. J. & Prentki, M. The islet β-cell: fuel responsive and vulnerable. Trends Endocrinol. Metab. 19, 285–291 (2008).
Prentki, M. & Nolan, C. J. Islet β cell failure in type 2 diabetes. J. Clin. Invest. 116, 1802–1812 (2006).
Bornfeldt, K. E. & Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 14, 575–585 (2011).
Imai, Y., Dobrian, A. D., Morris, M. A. & Nadler, J. L. Islet inflammation: a unifying target for diabetes treatment? Trends Endocrinol. Metab. 24, 351–360 (2013).
Johnson, A. M. & Olefsky, J. M. The origins and drivers of insulin resistance. Cell 152, 673–684 (2013).
Robertson, R. P. β-cell deterioration during diabetes: what's in the gun? Trends Endocrinol. Metab. 20, 388–393 (2009).
Saltiel, A. R. Insulin resistance in the defense against obesity. Cell Metab. 15, 798–804 (2012).
Samuel, V. T. & Shulman, G. I. Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871 (2012).
Gether, U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 21, 90–113 (2000).
Katritch, V., Cherezov, V. & Stevens, R. C. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 53, 531–556 (2013).
Kobilka, B. K. G protein coupled receptor structure and activation. Biochim. Biophys. Acta 1768, 794–807 (2007).
Claing, A., Laporte, S. A., Caron, M. G. & Lefkowitz, R. J. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog. Neurobiol. 66, 61–79 (2002).
Hall, R. A. & Lefkowitz, R. J. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ. Res. 91, 672–680 (2002).
Pierce, K. L. et al. Epidermal growth factor (EGF) receptor-dependent ERK activation by G protein-coupled receptors: a co-culture system for identifying intermediates upstream and downstream of heparin-binding EGF shedding. J. Biol. Chem. 276, 23155–23160 (2001).
Zhang, J. et al. Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization. Recept. Channels 5, 193–199 (1997).
Ahren, B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat. Rev. Drug Discov. 8, 369–385 (2009).
Berger, M. et al. Gαi/o-coupled receptor signaling restricts pancreatic β-cell expansion. Proc. Natl Acad. Sci. USA 112, 2888–2893 (2015).
Kim, H. et al. Serotonin regulates pancreatic β-cell mass during pregnancy. Nat. Med. 16, 804–808 (2010).
McArdle, M. A., Finucane, O. M., Connaughton, R. M., McMorrow, A. M. & Roche, H. M. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front. Endocrinol. (Lausanne) 4, 52 (2013).
Shoelson, S. E., Lee, J. & Goldfine, A. B. Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801 (2006).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Chawla, A., Nguyen, K. D. & Goh, Y. P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 11, 738–749 (2011).
Li, P. et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J. Biol. Chem. 285, 15333–15345 (2010).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Olefsky, J. M. & Glass, C. K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010).
Talukdar, S. et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 18, 1407–1412 (2012).
Winer, D. A. et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17, 610–617 (2011).
Gerner, R. R., Wieser, V., Moschen, A. R. & Tilg, H. Metabolic inflammation: role of cytokines in the crosstalk between adipose tissue and liver. Can. J. Physiol. Pharmacol. 91, 867–872 (2013).
Wieser, V., Moschen, A. R. & Tilg, H. Inflammation, cytokines and insulin resistance: a clinical perspective. Arch. Immunol. Ther. Exp. (Warsz) 61, 119–125 (2013).
Folco, G. & Murphy, R. C. Eicosanoid transcellular biosynthesis: from cell–cell interactions to in vivo tissue responses. Pharmacol. Rev. 58, 375–388 (2006).
Samuelsson, B. & Funk, C. D. Enzymes involved in the biosynthesis of leukotriene B4 . J. Biol. Chem. 264, 19469–19472 (1989).
Dixon, R. A. et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature 343, 282–284 (1990).
Peters-Golden, M. & Henderson, W. R. Jr. Leukotrienes. N. Engl. J. Med. 357, 1841–1854 (2007).
Radmark, O. & Samuelsson, B. 5-lipoxygenase: mechanisms of regulation. J. Lipid Res. 50, S40–S45 (2009).
Afonso, P. V. et al. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell 22, 1079–1091 (2012).
Kavelaars, A. et al. Increased acute inflammation, leukotriene B4-induced chemotaxis, and signaling in mice deficient for G protein-coupled receptor kinase 6. J. Immunol. 171, 6128–6134 (2003).
Rankin, J. A., Sylvester, I., Smith, S., Yoshimura, T. & Leonard, E. J. Macrophages cultured in vitro release leukotriene B4 and neutrophil attractant/activation protein (interleukin 8) sequentially in response to stimulation with lipopolysaccharide and zymosan. J. Clin. Invest. 86, 1556–1564 (1990).
Tian, W. et al. Blocking macrophage leukotriene B4 prevents endothelial injury and reverses pulmonary hypertension. Sci. Transl. Med. 5, 200ra117 (2013).
Tong, W. G. et al. Leukotriene B4 receptor antagonist LY293111 inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Clin. Cancer Res. 8, 3232–3242 (2002).
Yokomizo, T., Izumi, T., Chang, K., Takuwa, Y. & Shimizu, T. A. G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 387, 620–624 (1997).
Yokomizo, T., Kato, K., Terawaki, K., Izumi, T. & Shimizu, T. A second leukotriene B4 receptor, BLT2: a new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 192, 421–432 (2000).
Hill, A. T., Bayley, D. & Stockley, R. A. The interrelationship of sputum inflammatory markers in patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 160, 893–898 (1999).
Montuschi, P., Kharitonov, S. A., Ciabattoni, G. & Barnes, P. J. Exhaled leukotrienes and prostaglandins in COPD. Thorax 58, 585–588 (2003).
Chen, M. et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med. 203, 837–842 (2006).
Xu, S., Lu, H., Lin, J., Chen, Z. & Jiang, D. Regulation of TNFα and IL1β in rheumatoid arthritis synovial fibroblasts by leukotriene B4. Rheumatol. Int. 30, 1183–1189 (2010).
Beeh, K. M. et al. Neutrophil chemotactic activity of sputum from patients with COPD: role of interleukin 8 and leukotriene B4 . Chest 123, 1240–1247 (2003).
Crooks, S. W., Bayley, D. L., Hill, S. L. & Stockley, R. A. Bronchial inflammation in acute bacterial exacerbations of chronic bronchitis: the role of leukotriene B4. Eur. Respir. J. 15, 274–280 (2000).
Hicks, A., Monkarsh, S. P., Hoffman, A. F. & Goodnow, R. Jr. Leukotriene B4 receptor antagonists as therapeutics for inflammatory disease: preclinical and clinical developments. Expert Opin. Investig. Drugs 16, 1909–1920 (2007).
Nancey, S. et al. Blockade of LTB4 /BLT1 pathway improves CD8+ T-cell-mediated colitis. Inflamm. Bowel Dis. 17, 279–288 (2011).
Showell, H. J., Breslow, R., Conklyn, M. J., Hingorani, G. P. & Koch, K. Characterization of the pharmacological profile of the potent LTB4 antagonist CP-105,696 on murine LTB4 receptors in vitro. Br. J. Pharmacol. 117, 1127–1132 (1996).
Gronke, L. et al. Effect of the oral leukotriene B4 receptor antagonist LTB019 on inflammatory sputum markers in patients with chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 21, 409–417 (2008).
Spite, M. et al. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J. Immunol. 187, 1942–1949 (2011).
Li, P. et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat. Med. 21, 239–247 (2015).
González- Périz, A. et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by ω-3 fatty acids: a role for resolvins and protectins. Faseb J. 23, 1946–1957 (2009).
Proudman, S. M., Cleland, L. G. & James, M. J. Dietary omega-3 fats for treatment of inflammatory joint disease: efficacy and utility. Rheum. Dis. Clin. North Am. 34, 469–479 (2008).
Sijben, J. W. & Calder, P. C. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc. Nutr. Soc. 66, 237–259 (2007).
Calder, P. C. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br. J. Clin. Pharmacol. 75, 645–662 (2013).
Sorensen, L. S. et al. Rapid incorporation of ω-3 fatty acids into colonic tissue after oral supplementation in patients with colorectal cancer: a randomized, placebo-controlled intervention trial. J. Parenter. Enteral Nutr. 38, 617–624 (2013).
Sorensen, L. S. et al. Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. Br. J. Surg. 101, 33–42 (2014).
Yates, C. M., Calder, P. C. & Ed Rainger, G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 141, 272–282 (2014).
Yusof, H. M. et al. Limited impact of 2 g/day omega-3 fatty acid ethyl esters (Omacor®) on plasma lipids and inflammatory markers in patients awaiting carotid endarterectomy. Mar. Drugs 11, 3569–3581 (2013).
Cintra, D. E. et al. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS ONE 7, e30571 (2012).
Oh, D. Y. et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 (2010).
Williams-Bey, Y. et al. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS ONE 9, e97957 (2014).
Yan, Y. et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 38, 1154–1163 (2013).
Hirasawa, A. et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11, 90–94 (2005).
Gotoh, C. et al. The regulation of adipogenesis through GPR120. Biochem. Biophys. Res. Commun. 354, 591–597 (2007).
Christiansen, E. et al. Activity of dietary fatty acids on FFA1 and FFA4 and characterisation of pinolenic acid as a dual FFA1/FFA4 agonist with potential effect against metabolic diseases. Br. J. Nutr. 113, 1677–1688 (2015).
Burns, R. N. & Moniri, N. H. Agonism with the omega-3 fatty acids α-linolenic acid and docosahexaenoic acid mediates phosphorylation of both the short and long isoforms of the human GPR120 receptor. Biochem. Biophys. Res. Commun. 396, 1030–1035 (2010).
Hudson, B. D. et al. The pharmacology of TUG-891, a potent and selective agonist of the free fatty acid receptor 4 (FFA4/GPR120), demonstrates both potential opportunity and possible challenges to therapeutic agonism. Mol. Pharmacol. 84, 710–725 (2013).
Li, X., Yu, Y. & Funk, C. D. Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4). FASEB J. 27, 4987–4997 (2013).
Liu, Y. et al. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A2 via GPR120 receptor to produce prostaglandin E2 and plays an anti-inflammatory role in macrophages. Immunology 143, 81–95 (2014).
Liu, Z. et al. Omega-3 fatty acids and other FFA4 agonists inhibit growth factor signaling in human prostate cancer cells. J. Pharmacol. Exp. Ther. 352, 380–394 (2015).
Raptis, D. A. et al. GPR120 on Kupffer cells mediates hepatoprotective effects of ω3-fatty acids. J. Hepatol 60, 625–632 (2014).
Wellhauser, L. & Belsham, D. D. Activation of the omega-3 fatty acid receptor GPR120 mediates anti-inflammatory actions in immortalized hypothalamic neurons. J. Neuroinflamm. 11, 60 (2014).
Watson, S. J., Brown, A. J. & Holliday, N. D. Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol. Pharmacol. 81, 631–642 (2012).
Burns, R. N., Singh, M., Senatorov, I. S. & Moniri, N. H. Mechanisms of homologous and heterologous phosphorylation of FFA receptor 4 (GPR120): GRK6 and PKC mediate phosphorylation of Thr347, Ser350, and Ser357 in the C-terminal tail. Biochem. Pharmacol. 87, 650–659 (2014).
Ichimura, A. et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483, 350–354 (2012).
Bonnefond, A. et al. Contribution of the low-frequency, loss-of-function p.R270H mutation in FFAR4 (GPR120) to increased fasting plasma glucose levels. J. Med. Genet. 52, 595–598 (2015).
de Git, K. C. & Adan, R. A. Leptin resistance in diet-induced obesity: the role of hypothalamic inflammation. Obes. Rev. 16, 207–224 (2015).
Milanski, M. et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J. Neurosci. 29, 359–370 (2009).
Suckow, A. T. et al. Alteration of the glucagon axis in GPR120 (FFAR4) knockout mice: a role for GPR120 in glucagon secretion. J. Biol. Chem. 289, 15751–15763 (2014).
Yore, M. M. et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332 (2014).
Oh da, Y. et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 20, 942–947 (2014).
Meier, J. J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 8, 728–742 (2012).
Peters, A. Incretin-based therapies: review of current clinical trial data. Am. J. Med. 123, S28–S37 (2010).
Tzefos, M., Harris, K. & Brackett, A. Clinical efficacy and safety of once-weekly glucagon-like peptide-1 agonists in development for treatment of type 2 diabetes mellitus in adults. Ann. Pharmacother. 46, 68–78 (2012).
Fonseca, V. A. et al. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care 35, 1225–1231 (2012).
Grunberger, G. et al. Monotherapy with the once-weekly GLP-1 analogue dulaglutide for 12 weeks in patients with Type 2 diabetes: dose-dependent effects on glycaemic control in a randomized, double-blind, placebo-controlled study. Diabet. Med. 29, 1260–1267 (2012).
Cuthbertson, J., Patterson, S., O'Harte, F. P. & Bell, P. M. Addition of metformin to exogenous glucagon-like peptide-1 results in increased serum glucagon-like peptide-1 concentrations and greater glucose lowering in type 2 diabetes mellitus. Metabolism 60, 52–56 (2011).
Migoya, E. M. et al. Dipeptidyl peptidase-4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP-1. Clin. Pharmacol. Ther. 88, 801–808 (2010).
Zander, M., Taskiran, M., Toft-Nielsen, M. B., Madsbad, S. & Holst, J. J. Additive glucose-lowering effects of glucagon-like peptide-1 and metformin in type 2 diabetes. Diabetes Care 24, 720–725 (2001).
Vella, A. Common genetic variation influences the heterogeneity of response to oral glucose. Curr. Diab. Rep. 10, 249–251 (2010).
Drucker, D. J. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol. Endocrinol. 17, 161–171 (2003).
Drucker, D. J. The biology of incretin hormones. Cell Metab. 3, 153–165 (2006).
Drucker, D. J. The role of gut hormones in glucose homeostasis. J. Clin. Invest. 117, 24–32 (2007).
Ahren, B. & Schmitz, O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm. Metab. Res. 36, 867–876 (2004).
Brown, D. X. & Evans, M. Choosing between GLP-1 receptor agonists and DPP-4 inhibitors: a pharmacological perspective. J. Nutr. Metab. 2012, 381713 (2012).
Brunton, S. GLP-1 receptor agonists versus DPP-4 inhibitors for type 2 diabetes: is one approach more successful or preferable than the other? Int. J. Clin. Pract. 68, 557–567 (2014).
Unger, J. R. & Parkin, C. G. Glucagon-like peptide-1 (GLP-1) receptor agonists: differentiating the new medications. Diabetes Ther. 2, 29–39 (2011).
Dicker, D. DPP-4 inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes Care 34, S276–S278 (2011).
Doyle, M. E. & Egan, J. M. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 113, 546–593 (2007).
Sonoda, N. et al. β-arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic β cells. Proc. Natl Acad. Sci. USA 105, 6614–6619 (2008).
Deacon, C. F., Pridal, L., Klarskov, L., Olesen, M. & Holst, J. J. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am. J. Physiol. 271, E458–E464 (1996).
Hansen, L., Deacon, C. F., Orskov, C. & Holst, J. J. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140, 5356–5363 (1999).
Vilsboll, T., Agerso, H., Krarup, T. & Holst, J. J. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J. Clin. Endocrinol. Metab. 88, 220–224 (2003).
Farre, R. & Tack, J. Food and symptom generation in functional gastrointestinal disorders: physiological aspects. Am. J. Gastroenterol. 108, 698–706 (2013).
Richards, P. et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 63, 1224–1233 (2014).
Sisley, S. et al. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J. Clin. Invest. 124, 2456–2463 (2014).
Lamont, B. J. et al. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J. Clin. Invest. 122, 388–402 (2012).
Buteau, J., Foisy, S., Joly, E. & Prentki, M. Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 52, 124–132 (2003).
Wang, Q. & Brubaker, P. L. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia 45, 1263–1273 (2002).
Fehmann, H. C., Goke, R. & Goke, B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr. Rev. 16, 390–410 (1995).
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 (2007).
Orskov, C., Holst, J. J. & Nielsen, O. V. Effect of truncated glucagon-like peptide-1 [proglucagon-(78–107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology 123, 2009–2013 (1988).
Baynes, K. C. The evolving world of GLP-1 agonist therapies for type 2 diabetes. Ther. Adv. Endocrinol. Metab. 1, 61–67 (2010).
Secher, A. et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest. 124, 4473–4488 (2014).
Buse, J. B. et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann. Intern. Med. 154, 103–112 (2011).
Madsbad, S. Exenatide and liraglutide: different approaches to develop GLP-1 receptor agonists (incretin mimetics) — preclinical and clinical results. Best Pract. Res. Clin. Endocrinol. Metab. 23, 463–477 (2009).
Stonehouse, A., Walsh, B. & Cuddihy, R. Exenatide once-weekly clinical development: safety and efficacy across a range of background therapies. Diabetes Technol. Ther. 13, 1063–1069 (2011).
Freyse, E. J., Berg, S., Kohnert, K. D., Heinke, P. & Salzsieder, E. DPP-4 inhibition increases GIP and decreases GLP-1 incretin effects during intravenous glucose tolerance test in Wistar rats. Biol. Chem. 392, 209–215 (2011).
Kazafeos, K. Incretin effect: GLP-1, GIP, DPP4. Diabetes Res. Clin. Pract. 93, S32–S36 (2011).
Nikolaidis, L. A. et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 109, 962–965 (2004).
Read, P. A. et al. A pilot study to assess whether glucagon-like peptide-1 protects the heart from ischemic dysfunction and attenuates stunning after coronary balloon occlusion in humans. Circ. Cardiovasc. Interv. 4, 266–272 (2011).
Sokos, G. G. et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am. J. Cardiol. 100, 824–829 (2007).
Stranges, P. & Khanderia, U. Diabetes and cardiovascular disease: focus on glucagon-like peptide-1 based therapies. Ther. Adv. Drug Saf. 3, 185–201 (2012).
Crespin, S. R., Greenough, W. B. 3rd & Steinberg, D. Stimulation of insulin secretion by long-chain free fatty acids. A direct pancreatic effect. J. Clin. Invest. 52, 1979–1984 (1973).
Stein, D. T. et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J. Clin. Invest. 97, 2728–2735 (1996).
Briscoe, C. P. et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 278, 11303–11311 (2003).
Itoh, Y. et al. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 422, 173–176 (2003).
Latour, M. G. et al. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes 56, 1087–1094 (2007).
Stoddart, L. A., Brown, A. J. & Milligan, G. Uncovering the pharmacology of the G protein-coupled receptor GPR40: high apparent constitutive activity in guanosine 5′-O-(3-[35S]thio)triphosphate binding studies reflects binding of an endogenous agonist. Mol. Pharmacol. 71, 994–1005 (2007).
Poitout, V. & Robertson, R. P. Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocr. Rev. 29, 351–366 (2008).
Yaney, G. C. & Corkey, B. E. Fatty acid metabolism and insulin secretion in pancreatic beta cells. Diabetologia 46, 1297–1312 (2003).
Alquier, T. et al. Deletion of GPR40 impairs glucose-induced insulin secretion in vivo in mice without affecting intracellular fuel metabolism in islets. Diabetes 58, 2607–2615 (2009).
Kebede, M. et al. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes 57, 2432–2437 (2008).
Lan, H. et al. Lack of FFAR1/GPR40 does not protect mice from high-fat diet-induced metabolic disease. Diabetes 57, 2999–3006 (2008).
Nagasumi, K. et al. Overexpression of GPR40 in pancreatic β-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes 58, 1067–1076 (2009).
Vettor, R. et al. Loss-of-function mutation of the GPR40 gene associates with abnormal stimulated insulin secretion by acting on intracellular calcium mobilization. J. Clin. Endocrinol. Metab. 93, 3541–3550 (2008).
Doshi, L. S. et al. Acute administration of GPR40 receptor agonist potentiates glucose-stimulated insulin secretion in vivo in the rat. Metabolism 58, 333–343 (2009).
Luo, J. et al. A potent class of GPR40 full agonists engages the enteroinsular axis to promote glucose control in rodents. PLoS ONE 7, e46300 (2012).
Tan, C. P. et al. Selective small-molecule agonists of G protein-coupled receptor 40 promote glucose-dependent insulin secretion and reduce blood glucose in mice. Diabetes 57, 2211–2219 (2008).
Tsujihata, Y. et al. TAK-875, an orally available G protein-coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J. Pharmacol. Exp. Ther. 339, 228–237 (2011).
Ferdaoussi, M. et al. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia 55, 2682–2692 (2012).
Fujiwara, K., Maekawa, F. & Yada, T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet β-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am. J. Physiol. Endocrinol. Metab. 289, E670–E677 (2005).
Shapiro, H., Shachar, S., Sekler, I., Hershfinkel, M. & Walker, M. D. Role of GPR40 in fatty acid action on the β cell line INS-1E. Biochem. Biophys. Res. Commun. 335, 97–104 (2005).
Edfalk, S., Steneberg, P. & Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57, 2280–2287 (2008).
Flodgren, E. et al. GPR40 is expressed in glucagon producing cells and affects glucagon secretion. Biochem. Biophys. Res. Commun. 354, 240–245 (2007).
Wang, L. et al. Acute stimulation of glucagon secretion by linoleic acid results from GPR40 activation and [Ca2+]i increase in pancreatic islet α-cells. J. Endocrinol. 210, 173–179 (2011).
Burant, C. F. et al. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 379, 1403–1411 (2012).
Yashiro, H. et al. The effects of TAK-875, a selective G protein-coupled receptor 40/free fatty acid 1 agonist, on insulin and glucagon secretion in isolated rat and human islets. J. Pharmacol. Exp. Ther. 340, 483–489 (2012).
Hirasawa, A. et al. Production and characterization of a monoclonal antibody against GPR40 (FFAR1; free fatty acid receptor 1). Biochem. Biophys. Res. Commun. 365, 22–28 (2008).
Ma, D. et al. Expression of free fatty acid receptor GPR40 in the central nervous system of adult monkeys. Neurosci. Res. 58, 394–401 (2007).
Nakamoto, K. et al. Involvement of the long-chain fatty acid receptor GPR40 as a novel pain regulatory system. Brain Res. 1432, 74–83 (2012).
Kebede, M. et al. Glucose activates free fatty acid receptor 1 gene transcription via phosphatidylinositol-3-kinase-dependent O-GlcNAcylation of pancreas-duodenum homeobox-1. Proc. Natl Acad. Sci. USA 109, 2376–2381 (2012).
Mancini, A. D. & Poitout, V. The fatty acid receptor FFA1/GPR40 a decade later: how much do we know? Trends Endocrinol. Metab. 24, 398–407 (2013).
Li, Y. et al. β-cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes 54, 482–491 (2005).
Kalis, M. et al. Variants in the FFAR1 gene are associated with beta cell function. PLoS ONE 2, e1090 (2007).
Dupuis, J. et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 42, 105–116 (2010).
Wagner, R. et al. Reevaluation of fatty acid receptor 1 as a drug target for the stimulation of insulin secretion in humans. Diabetes 62, 2106–2111 (2013).
Negoro, N. et al. Discovery of TAK-875: a potent, selective, and orally bioavailable GPR40 agonist. ACS Med. Chem. Lett. 1, 290–294 (2010).
Kaku, K., Araki, T. & Yoshinaka, R. Randomized, double-blind, dose-ranging study of TAK-875, a novel GPR40 agonist, in Japanese patients with inadequately controlled type 2 diabetes. Diabetes Care 36, 245–250 (2013).
Defossa, E. & Wagner, M. Recent developments in the discovery of FFA1 receptor agonists as novel oral treatment for type 2 diabetes mellitus. Bioorg. Med. Chem. Lett. 24, 2991–3000 (2014).
Lin, D. C. et al. Identification and pharmacological characterization of multiple allosteric binding sites on the free fatty acid 1 receptor. Mol. Pharmacol. 82, 843–859 (2012).
Xiong, Y. et al. Activation of FFA1 mediates GLP-1 secretion in mice. Evidence for allosterism at FFA1. Mol. Cell Endocrinol. 369, 119–129 (2013).
Lin, D. C. et al. AMG 837: a novel GPR40/FFA1 agonist that enhances insulin secretion and lowers glucose levels in rodents. PLoS ONE 6, e27270 (2011).
Chu, Z. L. et al. A role for intestinal endocrine cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology 149, 2038–2047 (2008).
Chu, Z. L. et al. A role for β-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology 148, 2601–2609 (2007).
Parker, H. E., Habib, A. M., Rogers, G. J., Gribble, F. M. & Reimann, F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 52, 289–298 (2009).
Reimann, F. et al. Glucose sensing in L cells: a primary cell study. Cell Metab. 8, 532–539 (2008).
Hansen, K. B. et al. 2-oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J. Clin. Endocrinol. Metab. 96, E1409–E1417 (2011).
Overton, H. A. et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 3, 167–175 (2006).
Soga, T. et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 326, 744–751 (2005).
Hansen, H. S., Rosenkilde, M. M., Holst, J. J. & Schwartz, T. W. GPR119 as a fat sensor. Trends Pharmacol. Sci. 33, 374–381 (2012).
Lauffer, L., Iakoubov, R. & Brubaker, P. L. GPR119: 'double-dipping' for better glycemic control. Endocrinology 149, 2035–2037 (2008).
Lan, H. et al. GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J. Endocrinol. 201, 219–230 (2009).
Soga, T. et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 326, 744–751 (2005).
Jones, R. M., Leonard, J. N., Buzard, D. J. & Lehmann, J. GPR119 agonists for the treatment of type 2 diabetes. Expert Opin. Ther. Pat. 19, 1339–1359 (2009).
Shah, U. & Kowalski, T. J. GPR119 agonists for the potential treatment of type 2 diabetes and related metabolic disorders. Vitam. Horm. 84, 415–448 (2010).
Moran, B. M., Abdel-Wahab, Y. H., Flatt, P. R. & McKillop, A. M. Activation of GPR119 by fatty acid agonists augments insulin release from clonal β-cells and isolated pancreatic islets and improves glucose tolerance in mice. Biol. Chem. 395, 453–464 (2014).
Lee, Y. S. et al. The fractalkine/CX3CR1 system regulates β cell function and insulin secretion. Cell 153, 413–425 (2013).
Aoyama, T., Inokuchi, S., Brenner, D. A. & Seki, E. CX3CL1–CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 52, 1390–1400 (2010).
Haskell, C. A., Cleary, M. D. & Charo, I. F. Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction. Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. J. Biol. Chem. 274, 10053–10058 (1999).
Lucas, A. D. et al. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am. J. Pathol. 158, 855–866 (2001).
Combadiere, C. et al. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation 107, 1009–1016 (2003).
Imai, T. et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91, 521–530 (1997).
Zernecke, A., Shagdarsuren, E. & Weber, C. Chemokines in atherosclerosis: an update. Arterioscler. Thromb. Vasc. Biol. 28, 1897–1908 (2008).
Cardona, A. E. et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9, 917–924 (2006).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Inoue, A. et al. Antagonist of fractalkine (CX3CL1) delays the initiation and ameliorates the progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum. 52, 1522–1533 (2005).
Garton, K. J. et al. Tumor necrosis factor-α-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J. Biol. Chem. 276, 37993–38001 (2001).
Hundhausen, C. et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell–cell adhesion. Blood 102, 1186–1195 (2003).
Shah, R. et al. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes 60, 1512–1518 (2011).
Sirois-Gagnon, D. et al. Association of common polymorphisms in the fractalkine receptor (CX3CR1) with obesity. Obes. (Silver Spring) 19, 222–227 (2011).
Cnop, M. Fatty acids and glucolipotoxicity in the pathogenesis of Type 2 diabetes. Biochem. Soc. Trans. 36, 348–352 (2008).
van Raalte, D. H. & Diamant, M. Glucolipotoxicity and beta cells in type 2 diabetes mellitus: target for durable therapy? Diabetes Res. Clin. Pract. 93, S37–S46 (2011).
Donath, M. Y., Dalmas, E., Sauter, N. S. & Boni-Schnetzler, M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab. 17, 860–872 (2013).
Eguchi, K. & Manabe, I. Macrophages and islet inflammation in type 2 diabetes. Diabetes Obes. Metab. 15, 152–158 (2013).
Ehses, J. A., Ellingsgaard, H., Boni-Schnetzler, M. & Donath, M. Y. Pancreatic islet inflammation in type 2 diabetes: from α and β cell compensation to dysfunction. Arch. Physiol. Biochem. 115, 240–247 (2009).
Mancini, A. D. & Poitout, V. GPR40 agonists for the treatment of type 2 diabetes: life after 'TAKing' a hit. Diabetes Obes. Metab. 17, 622–629 (2015).
Acknowledgements
The authors' work is funded in part by NIH grants to J.M.O. (DK033651, DK074868, DK063491, DK09062), and an American Heart Association Scientist Development Grant to D.Y.O. (14SDG19880020). We thank Angela Tyler for editorial assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Oh, D., Olefsky, J. G protein-coupled receptors as targets for anti-diabetic therapeutics. Nat Rev Drug Discov 15, 161–172 (2016). https://doi.org/10.1038/nrd.2015.4
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2015.4
This article is cited by
-
The G protein coupled receptor CXCR4 designed by the QTY code becomes more hydrophilic and retains cell signaling activity
Scientific Reports (2020)
-
Density functional theory calculations of spectral, NLO, reactivity, NBO properties and docking study of Vincosamide-N-Oxide active against lung cancer cell lines H1299
SN Applied Sciences (2020)
-
Adipocyte β-arrestin-2 is essential for maintaining whole body glucose and energy homeostasis
Nature Communications (2019)
-
GPCR drug discovery: integrating solution NMR data with crystal and cryo-EM structures
Nature Reviews Drug Discovery (2019)
-
A Narrative Review of Potential Future Antidiabetic Drugs: Should We Expect More?
Indian Journal of Clinical Biochemistry (2018)