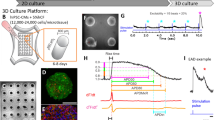
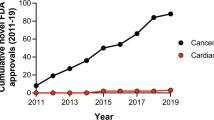
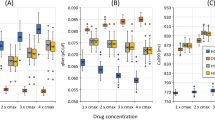
Abstract
The early and efficient assessment of cardiac safety liabilities is essential to confidently advance novel drug candidates. This article discusses evolving mechanistically based preclinical strategies for detecting drug-induced electrophysiological and structural cardiotoxicity using in vitro human ion channel assays, human-based in silico reconstructions and human stem cell-derived cardiomyocytes. These strategies represent a paradigm shift from current approaches, which rely on simplistic in vitro assays that measure blockade of the Kv11.1 current (also known as the hERG current or IKr) and on the use of non-human cells or tissues. These new strategies have the potential to improve sensitivity and specificity in the early detection of genuine cardiotoxicity risks, thereby reducing the likelihood of mistakenly discarding viable drug candidates and speeding the progression of worthy drugs into clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dessertenne, F. La tachycardie ventriculaire a deux foyers opposes variables. Arch. Mal. Coeur Vaiss. 59, 263–272 (in French) (1966).
Fabiato, A. & Coumel, P. Torsades de pointes, a quarter of a century later: a tribute to Dr. F. Dessertenne. Cardiovasc. Drugs. Ther. 5, 167–169 (1991).
Kannankeril, P., Roden, D. M. & Darbar, D. Drug-induced long QT syndrome. Pharmacol. Rev. 62, 760–781 (2010).
Roden, D. M. Cellular basis of drug-induced torsades de pointes. Br. J. Pharm. 154, 1502–1507 (2008).
Sanguinetti, M. C., Jiang, C., Curran, M. E. & Keating, M. T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81, 299–307 (1995).
Sanguinetti, M. C. HERG1 channelopathies. Pflugers Arch. 460, 265–276 (2010).
Rampe, D. & Brown, A. M. A history of the role of the hERG channel in cardiac risk assessment. J. Pharmacol. Toxicol. Methods. 68, 13–22 (2013).
Vandenberg, J. I. et al. hERG K+ channels: structure, function, and clinical significance. Physiol. Rev. 92, 1393–1478 (2012).
Hasinoff, B. B. & Patel, D. The lack of target specificity of small molecule anticancer kinase inhibitors is correlated with their ability to damage myocytes in vitro. Toxicol. Appl. Pharmacol. 249, 132–139 (2010).
Cheng, H. & Force, T. Why do kinase inhibitors cause cardiotoxicity and what can be done about it? Prog. Cardiovasc. Dis. 53, 114–120 (2010).
Greineder, C. F., Kohnstamm, S. & Ky, B. Heart failure associated with sunitinib: lessons learned from animal models. Curr. Hypertens. Rep. 13, 436–441 (2011).
Cross, M. J. et al. Physiological, pharmacological and toxicological considerations of drug-induced structural cardiac injury. Br. J. Pharmacol. 172, 957–974 (2015).
Chi, K. R. Revolution dawning in cardiotoxicity testing. Nat. Rev. Drug Discov. 12, 565–567 (2013).
Sager, P. T., Gintant, G., Turner, R., Pettit, S. & Stockbridge, N. Rechanneling the cardiac proarrhythmia safety paradigm: a meeting report from the Cardiac Safety Research Consortium. Am. Heart J. 167, 292–300 (2014).
Stockbridge, N., Morganroth, J., Shah, R. R. & Garnett, C. Dealing with global safety issues: was the response to QT-liability of non-cardiac drugs well coordinated? Drug Saf. 36, 167–182 (2013).
Committee for Propprietary Medicinal Products. Points to consider: the assessment of the potential for QT interval prolongation by non-cardiovsacular medicinal products. FDA [online], (1997).
Woosley, R. L., Chen, Y., Freiman, J. P. & Gillis, R. A. Mechanism of the cardiotoxic actions of terfenadine. JAMA 269, 1532–1536 (1993).
Spector, P. S., Curran, M. E., Keating, M. T. & Sanguinetti, M. C. Class III antiarrhythmic drugs block HERG, a human cardiac delayed rectifier K+ channel. Open-channel block by methanesulfonanilides. Circ. Res. 78, 499–503 (1996).
Curran, M. E. et al. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 80, 795–803 (1995).
U.S. Department of Health and Human Services. The non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval Prolongation) by human pharmaceuticals S7B. FDA [online], (2005).
U.S. Department of Health and Human Services. E14: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. FDA [online], (2005).
Cavero, I. Safety Pharmacology Society: 8th annual meeting. Expert Opin. Drug Saf. 8, 237–247 (2009).
Lindgren, S. et al. Benchmarking safety pharmacology regulatory packages and best practice. J. Pharmacol. Toxicol. Methods. 58, 99–109 (2008).
Bowes, J. et al. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat. Rev. Drug Discov. 11, 909–922 (2012).
De Ponti, F. in Antitargets: Prediction and Prevention of Drug Side Effects (eds Vaz, R. J. & Klabunde, T.) 55–88 (Wiley-VCH, 2008).
Gintant, G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol. Ther. 129, 109–119 (2011).
Kramer, J. et al. Mice models: superior to the HERG model in predicting torsade de pointes. Nat. Sci. Rep. 3, 2100 (2013).
Lacerda, A. E. et al. Alfuzosin delays cardiac repolarization by a novel mechanism. J. Pharmacol. Exp. Ther. 324, 427–433 (2008).
Tzeis, S. & Andrikopoulos, G. Antiarrhythmic properties of ranolazine — from bench to bedside. Expert Opin. Investig. Drugs 21, 1733–1741 (2012).
Mirams, G. R. et al. Prediction of thorough QT study results using action potential simulations based on ion channel screens. J. Pharmacol. Toxicol. Methods. 70, 246–254 (2014).
Roden, D. M. Taking the 'idio' out of 'idiosyncratic': predicting torsades de pointes. Pacing Clin. Electrophysiol. 21, 1029–1034 (1998).
Varró, A. & Baczkó, I. Cardiac ventricular repolarization reserve: a principle for understanding drug-related proarrhythmic risk. Br. J. Pharmacol. 164, 14–36 (2011).
Bril, A. et al. Combined potassium and calcium channel blocking activities as the basis for antiarrhythmic properties with low proarrhythmic risk: experimental antiarrhythmic effect of BRL-32872. J. Pharmacol. Exp. Ther. 276, 637–646 (1996).
Martin, R. L. et al. The utility of hERG and repolarization assays in evaluating delayed cardiac repolarization: influence of multi-channel block. J. Cardiovasc. Pharmacol. 43, 369–379 (2004).
Zhang, S., Zhou, Z., Gong, Q., Makielski, J. C. & January, C. T. Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ. Res. 84, 989–998 (1999).
Sager, P. T. Key clinical considerations for demonstrating the utility of preclinical models to predict clinical drug-induced torsades de pointes. Br. J. Pharmacol. 154, 1544–1549 (2008).
Wu, L. et al. Reduction of repolarization reserve unmasks the proarrhythmic role of endogenous late Na+ current in the heart. Am. J. Physiol. Heart Circ. Physiol. 297, H1048–H1057 (2009).
Janse, M. J. et al. Repolarization gradients in the intact heart: transmural or apico-basal? Prog. Biophys. Mol. Biol. 109, 6–15 (2012).
Sadrieh, A. et al. Quantifying the origins of population variability in cardiac electrical activity through sensitivity analysis of the electrocardiogram. J. Physiol. 591, 4207–4222 (2013).
Vijayakumar, R. et al. Electrophysiologic substrate in congenital long QT syndrome: noninvasive mapping with electrocardiographic imaging (ECGI). Circulation 130, 1936–1943 (2014).
Redfern, W. S. et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 58, 32–45 (2003).
Wallis, R. M. Integrated risk assessment and predictive value to humans of non-clinical repolarization assays. Br. J. Pharmacol. 159, 115–121 (2010).
Deeks, J. J. & Altman, D. G. Diagnostic tests 4: likelihood ratios. BMJ 329, 168–169 (2004).
O'Hara, T. & Rudy, Y. Quantitative comparison of cardiac ventricular myocyte electrophysiology and response to drugs in human and nonhuman species. Am. J. Physiol. Heart Circ. Physiol. 302, H1023–H1030 (2012).
Husti, Z. et al. Combined inhibition of key potassium currents has different effects on cardiac repolarization reserve and arrhythmia susceptibility in dogs and rabbits. Can. J. Physiol. Pharmacol. 4, 1–10 (2015).
Lu, H. R., Mariën, R., Saels, A. & De Clerck, F. Species plays an important role in drug-induced prolongation of action potential duration and early afterdepolarizations in isolated Purkinje fibers. J. Cardiovasc. Electrophysiol. 12, 93–102 (2001).
Wu, Y., Carlsson, L., Liu, T., Kowey, P. R. & Yan, G. X. Assessment of the proarrhythmic potential of the novel antiarrhythmic agent AZD7009 and dofetilide in experimental models of torsades de pointes. J. Cardiovasc. Electrophysiol. 16, 898–904 (2005).
Liu, T. et al. Blinded validation of the isolated arterially perfused rabbit ventricular wedge in preclinical assessment of drug-induced proarrhythmias. Heart Rhythm 3, 948–956 (2006).
Chen, X., Cordes, J. S., Bradley, J. A., Sun, Z. & Zhou, J. Use of arterially perfused rabbit ventricular wedge in predicting arrhythmogenic potentials of drugs. J. Toxicol. Methods 54, 261–272 (2006).
Hondeghem, L. M., Carlsson, L. & Duker, G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation 103, 2004–2013 (2001).
Hondeghem, L. M. & Hoffmann, P. Blinded test in isolated female rabbit heart reliably identifies action potential duration prolongation and proarrhythmic drugs: importance of triangulation, reverse use dependence, and instability. J. Cardiovasc. Pharmacol. 41, 14–24 (2003).
Lawrence, C. L., Bridgland-Taylor, M. H., Pollard, C. E., Hammond, T. G. & Valentin, J. P. A rabbit Langendorff heart proarrhythmia model: predictive value for clinical identification of torsades de pointes. Br. J. Pharmacol. 149, 845–860 (2006).
Jost, N. et al. Ionic mechanisms limiting cardiac repolarization-reserve in humans compared to dogs. J. Physiol. 591, 4189–4206 (2013).
Chain, A. S. et al. Identifying the translational gap in the evaluation of drug-induced QTc interval prolongation. Br. J. Clin. Pharmacol. 76, 708–724 (2013).
Vos, M. A., van Opstal, J. M., Leunissen, J. D. & Verduyn, S. C. Electrophysiologic parameters and predisposing factors in the generation of drug-induced torsade de pointes arrhythmias. Pharmacol. Ther. 92, 109–122 (2001).
Sato, T., Hirao, K. & Hiejima, K. The relationship between early afterdepolarization and the occurrence of torsades de pointes — an in vivo canine model study. Jpn Circ. J. 57, 543–552 (1993).
Vos, M. A., Verduyn, S. C., Gorgels, A. P., Lipcsei, G. C. & Wellens, H. J. Reproducible induction of early after depolarizations and torsade de pointes arrhythmias by d-sotalol and pacing in dogs with chronic atrioventricular block. Circulation 91, 864–872 (1995).
Roden, D. M. Early after-depolarizations and torsade de pointes: implications for the control of cardiac arrhythmias by prolonging repolarization. Eur Heart J. 14(Suppl. H), 56–61 (1993).
Poelzing, S. & Rosenbaum, D. S. Cellular mechanisms of torsade de pointes. Novartis Found. Symp. 266, 204–217 (2005).
Huffaker, R. B., Weiss, J. N. & Kogan, B. Effects of early afterdepolarizations on reentry in cardiac tissue: a simulation study. Am. J. Physiol. Heart Circ. Physiol. 292, H3089–H3102 (2007).
Vandersickel, N. et al. A study of early afterdepolarizations in a model for human ventricular tissue. PLoS ONE 9, e84595 (2014).
Starmer, C. F. Characterizing EAD Likelihood with a safety factor or the proarrhythmic potential of slowed repolarization and early afterdepolarizations (EADs). itlab [online], (2005).
Viswanathan, P. C. & Rudy, Y. Pause induced early afterdepolarizations in the long QT syndrome: a simulation study. Cardiovasc. Res. 42, 530–542 (1999).
Qu, Z. et al. Early afterdepolarizations in cardiac myocytes: beyond reduced repolarization reserve. Cardiovasc. Res. 99, 6–15 (2013).
Chi, K. R. Revolution dawning in cardiotoxicity testing. Nat. Rev. Drug Discov. 12, 565–567 (2013).
Sager, P. T., Gintant, G., Turner, J. R., Pettit, S. & Stockbridge, N. Rechanneling the cardiac proarrhythmia safety paradigm: a meeting report from the Cardiac Safety Research Consortium. Am. Heart J. 167, 292–300 (2014).
Farre, C. & Fertig, N. HTS techniques for patch clamp-based ion channel screening — advances and economy. Expert Opin. Drug Discov. 7, 515–524 (2012).
Corrias, A. et al. Arrhythmic risk biomarkers for the assessment of drug cardiotoxicity: from experiments to computer simulations. Philos. Trans. A Math. Phys. Eng. Sci. 368, 3001–3025 (2010).
Romero, L., Trenor, B., Yang, P. C., Saiz, J. & Clancy, C. E. In silico screening of the impact of hERG channel kinetic abnormalities on channel block and susceptibility to acquired long QT syndrome. J. Mol. Cell Cardiol. 72, 126–137 (2014).
Veroli, D. I. et al. hERG inhibitors with similar potency but different binding kinetics do not pose the same proarrhythmic risk: implications for drug safety assessment. J. Cardiovasc. Electrophysiol. 25, 197–207 (2014).
Polak, S., Wis´niowska, B. & Brandys, J. Collation, assessment and analysis of literature in vitro data on hERG receptor blocking potency for subsequent modeling of drugs' cardiotoxic properties. J. Appl. Toxicol. 29, 183–206 (2009).
Danker, T. & Möller, C. Early identification of hERG liability in drug discovery programs by automated patch clamp. Front. Pharmacol. 5, 203 (2014).
Brimecombe, J. C., Kirsch, G. E. & Brown, A. M. Test article concentrations in the hERG assay: losses through the perfusion, solubility and stability. J. Pharmacol. Toxicol. Methods 59, 29–34 (2009).
Kirsch, G. E. et al. Variability in the measurement of hERG potassium channel inhibition: effects of temperature and stimulus pattern. J. Pharmacol. Toxicol. Methods 50, 93–101 (2004).
Milnes, J. T., Witchel, H. J., Leaney, J. L., Leishman, D. J. & Hancox, J. C. Investigating dynamic protocol-dependence of hERG potassium channel inhibition at 37 °C: cisapride versus dofetilide. J. Pharmacol. Toxicol. Methods 61, 178–191 (2010).
Margulis, M. et al. Protein binding-dependent decreases in hERG channel blocker potency assessed by whole-cell voltage clamp in serum. J. Cardiovasc. Pharmacol. 55, 368–376 (2010).
ten Tusscher, K. H., Noble, D., Noble, P. J. & Panfilov, A. V. A model for human ventricular tissue. Am. J. Physiol. Heart Circ. Physiol. 286, H1573–H1589 (2004).
O'Hara, T., Virág, L., Varró, A. & Rudy, Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput. Biol. 7, e1002061 (2011).
Zemzemi, N. et al. Computational assessment of drug-induced effects on the electrocardiogram: from ion channel to body surface potentials. Br. J. Pharmacol. 168, 718–733 (2013).
Okada, J. et al. Screening system for drug-induced arrhythmogenic risk combining a patch clamp and heart simulator. Sci. Adv. 1, e1400142 (2015).
Mirams, G. R., Davies, M. R., Cui, Y., Kohl, P. & Noble, D. Application of cardiac electrophysiology simulations to pro-arrhythmic safety testing. Br. J. Pharmacol. 167, 932–945 (2012).
Elshrif, M. M. & Cherry, E. M. A quantitative comparison of the behavior of human ventricular cardiac electrophysiology models in tissue. PLoS ONE 9, e84401 (2014).
Sarkar, A. X. & Sobie, E. A. Regression analysis for constraining free parameters in electrophysiological models of cardiac cells. PLoS Comput. Biol. 6, e1000914 (2010).
Sarker, A. X. & Sobie, E. A. Quantification of repolarization reserve to understand interpatient variability in the response to pro-arrhythmic drugs: a computational analysis. Heart Rhythm 8, 1749–1755 (2011).
Groenendaal, W. et al. Cell-specific cardiac electrophysiology models. PLoS Comput. Biol. 11, e1004242 (2015).
Beattie, K. A. et al. Evaluation of an in silico cardiac safety assay: using ion channel screening data to predict QT interval changes in the rabbit ventricular wedge. J. Pharmacol. Toxicol. Methods 68, 88–96 (2013).
Mirams, G. R. et al. Simulation of multiple ion channel block provides improved early prediction of compounds' clinical torsadogenic risk. Cardiovasc. Res. 91, 53–61 (2011).
Romero, L., Pueyo, E., Fink, M. & Rodríguez, B. Impact of ionic current variability on human ventricular cellular electrophysiology. Am. J. Physiol. Heart Circ. Physiol. 297, H1436–H1445 (2009).
Di Veroli, G. Y., Davies, M. R., Zhang, H., Abi-Gerges, N. & Boyett, M. R. High-throughput screening of drug-binding dynamics to HERG improves early drug safety assessment. Am. J. Physiol. Heart Circ. Physiol. 304, H104–H117 (2013).
Moreno, J. D. et al. Ranolazine for congenital and acquired late INa-linked arrhythmias: in silico pharmacological screening. Circ. Res. 113, e50–e61 (2013).
Trenor, B. et al. In silico assessment of drug safety in human heart applied to late sodium current blockers. Channels (Austin) 7, 249–262 (2013).
Chang, M. G. et al. Dynamics of early afterdepolarization-mediated triggered activity in cardiac monolayers. Biophys. J. 102, 2706–2714 (2012).
Qu, Y., Gao, B., Fang, M. & Vargas, H. M. Human embryonic stem cell derived cardiac myocytes detect hERG-mediated repolarization effects, but not Nav1.5 induced depolarization delay. J. Pharmacol. Toxicol. Methods. 68, 74–81 (2013).
Wagner, S., Maier, L. S. & Bers, D. M. Role of sodium and calcium dysregulation in tachyarrhythmias in sudden cardiac death. Circ. Res. 116, 1956–1970 (2015).
Heijman, J. et al. Determinants of beat-to-beat variability of repolarization duration in the canine ventricular myocyte: a computational analysis. PLoS Comput. Biol. 9, e1003202 (2013).
Christophe, B. Simulation of early after-depolarization in non-failing human ventricular myocytes: can this help cardiac safety pharmacology? Pharmacol. Rep. 365, 1281–1293 (2013).
Grskovic, M., Javaherian, A., Strulovici, B. & Daley, G. Q. Induced pluripotent stem cells — opportunities for disease modelling and drug discovery. Nat. Rev. Drug Discov. 10, 915–929 (2011).
Dick, E., Rajamohan, D., Ronksley, J. & Denning, C. Evaluating the utility of cardiomyocytes from human pluripotent stem cells for drug screening. Biochem. Soc. Trans. 38, 1037–1045 (2010).
Matsa, E. & Denning, C. In vitro uses of human pluripotent stem cell-derived cardiomyocytes. J. Cardiovasc. Transl. Res. 5, 581–592 (2012).
Rajamohan, D. et al. Current status of drug screening and disease modelling in human pluripotent stem cells. Bioessays 35, 281–298 (2013).
Hoekstra, M., Mummery, C. L., Wilde, A. A., Bezzina, C. R. & Verkerk, A. O. Induced pluripotent stem cell derived cardiomyocytes as models for cardiac arrhythmias. Front. Physiol. 3, 346 (2012).
Jeong, E. M. et al. Metabolic stress, reactive oxygen species, and arrhythmia. J. Mol. Cell. Cardiol. 52, 454–463 (2012).
Karagueuzian, H. S., Nguyen, T. P., Qu, Z. & Weiss, J. N. Oxidative stress, fibrosis, and early afterdepolarization-mediated cardiac arrhythmias. Front. Physiol. 4, 19 (2013).
Hille, B., Dickson, E., Kruse, M. & Falkenburger, B. Dynamic metabolic control of an ion channel. Prog. Mol. Biol. Transl. Sci. 123, 219–247 (2014).
Heath, B. M. & Terrar, D. A. Protein kinase C enhances the rapidly activating delayed rectifier potassium current, IKr, through a reduction in C-type inactivation in guinea-pig ventricular myocytes. J. Physiol. 522, 391–402 (2000).
Cockerill, S. L. et al. Modulation of hERG potassium currents in HEK-293 cells by protein kinase C. Evidence for direct phosphorylation of pore forming subunits. J. Physiol. 581, 479–493 (2007).
Mewe, M. et al. Modulation of cardiac ERG1 K+ channels by cGMP signaling. J. Mol. Cell. Cardiol. 49, 48–57 (2010).
Yang, T. et al. Screening for acute IKr block is insufficient to detect torsades de pointes liability: role of late sodium current. Circulation 130, 224–234 (2014).
Ballou, L. M., Lin, R. Z. & Cohen, I. S. Control of cardiac repolarization by phosphoinositide 3-kinase signaling to ion channels. Circ. Res. 16, 127–137 (2015).
Kraushaar, U. et al. Cardiac safety pharmacology: from human ether-a-gogo related gene channel block towards induced pluripotent stem cell based disease models. Expert Opin. Drug Saf. 11, 285–298 (2012).
Scott, C. W., Peters, M. F. & Dragan, Y. P. Human induced pluripotent stem cells and their use in drug discovery for toxicity testing. Toxicol. Lett. 219, 49–58 (2013).
Scheel, O. et al. Action potential characterization of human induced pluripotent stem cell-derived cardiomyocytes using automated patch-clamp technology. Assay Drug Dev. Technol. 12, 457–469 (2014).
Nakamura, Y. et al. Assessment of testing methods for drug-induced repolarization delay and arrhythmias in an iPS cell-derived cardiomyocyte sheet: multi-site validation study. J. Pharmacol. Sci. 124, 494–501 (2014).
Sekino, Y., Sato, K., Kanda, Y. & Ishida, S. Developing and standardizing experimental protocols using human iPS-derived cells to predict adverse drug reactions in pre-clinical safety studies. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku 131, 25–34 (in Japanese) (2013).
Gorospe, G., Younes, L., Tung, L. & Vidal, R. A metamorphosis distance for embryonic cardiac action potential interpolation and classification. Med. Image Comput. Comput. Assist. Interv. 16, 469–476 (2013).
Ivashchenko, C. Y. et al. Human-induced pluripotent stem cell-derived cardiomyocytes exhibit temporal changes in phenotype. Am. J. Physiol. Heart Circ. Physiol. 305, H913–H922 (2013).
Sheehy, S. P. et al. Quality metrics for stem cell-derived cardiac myocytes. Stem Cell Reports 2, 282–294 (2014).
Ma, J. et al. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 301, H2006–H2017 (2011).
Honda, M., Kiyokawa, J., Tabo, M. & Inoue, T. Electrophysiological characterization of cardiomyocytes derived from human induced pluripotent stem cells. J. Pharmacol. Sci. 117, 149–159 (2011).
Jonsson, M. K. et al. Application of human stem cell-derived cardiomyocytes in safety pharmacology requires caution beyond hERG. J Mol. Cell. Cardiol. 52, 998–1008 (2012).
van den Heuvel, N. H., van Veen, T. A., Lim, B. & Jonsson, M. K. Lessons from the heart: mirroring electrophysiological characteristics during cardiac development to in vitro differentiation of stem cell derived cardiomyocytes. J. Mol. Cell. Cardiol. 67, 12–25 (2013).
Peng, S., Lacerda, A. E., Kirsch, G. E., Brown, A. M. & Bruening-Wright, A. The action potential and comparative pharmacology of stem cell-derived human cardiomyocytes. J. Pharmacol. Toxicol. Methods 61, 277–286 (2010).
Qu, Y., Gao, B., Fang, M. & Vargas, H. M. Human embryonic stem cell derived cardiac myocytes detect hERG-mediated repolarization effects, but not Nav1.5 induced depolarization delay. J. Pharmacol. Toxicol. Methods 68, 74–81 (2013).
Gibson, J. K., Yue, Y., Bronson, J., Palmer, C. & Numann, R. Human stem cell-derived cardiomyocytes detect drug-mediated changes in action potentials and ion currents. J. Pharmacol. Toxicol. Methods 70, 255–267 (2014).
Harris, K. et al. Comparison of electrophysiological data from human-induced pluripotent stem cell-derived cardiomyocytes to functional preclinical safety assays. Toxicol. Sci. 134, 412–426 (2013).
Navarrete, E. G. et al. Screening drug-induced arrhythmia [corrected] using human induced pluripotent stem cell-derived cardiomyocytes and low-impedance microelectrode arrays. Circulation 128, S3–S13 (2013).
Clements, M. & Thomas, N. High-throughput multi-parameter profiling of electrophysiological drug effects in human embryonic stem cell derived cardiomyocytes using multi-electrode arrays. Toxicol. Sci. 140, 445–461 (2014).
Hirt, M. N. et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J. Mol. Cell. Cardiol. 74, 151–161 (2014).
Novakovic, G. V., Eschenhagen, T. & Mummery, C. Myocardial tissue engineering: in vitro models. Cold Spring Harb. Perspect. Med. 4, a014076 (2014).
Lundy, S. D., Zhu, W. Z., Regnier, M. & Laflamme, M. A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 22, 1991–2002 (2013).
Ribeiro, M. C. et al. Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro — correlation between contraction force and electrophysiology. Biomaterials 51, 138–150 (2015).
Kuryshev, Y. A. et al. Pentamidine-induced long QT syndrome and block of hERG trafficking. J Pharmacol Exp Ther. 312, 316–323 (2005).
Tanaka, H. et al. Effect of terfenadine and pentamidine on the HERG channel and its intracellular trafficking: combined analysis with automated voltage clamp and confocal microscopy. Biol. Pharm. Bull. 37, 1826–1830 (2014).
Davis, R. P. et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation 125, 3079–3091 (2012).
Liang, P. et al. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation 127, 1677–1691 (2013).
Horackova, M. Excitation−contraction coupling in isolated adult ventricular myocytes from the rat, dog, and rabbit: effects of various inotropic interventions in the presence of ryanodine. Can. J. Physiol. Pharmacol. 64, 1473–1483 (1986).
Bers, D. M., Bassani, J. W. M. & Bassani, R. A. Na-Ca exchange and Ca fluxes during contraction and relaxation in mammalian ventricular muscle. Ann. NY Acad. Sci. 779, 430–442 (1996).
Milani-Nejad, N. & Janssen, P. M. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol. Ther. 141, 235–249 (2014).
Lee, Y. K. et al. Calcium homeostasis in human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Rev. 7, 976–986 (2011).
Lieu, D. K. et al. Absence of transverse tubules contributes to non-uniform Ca2+ wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev. 18, 1493–1500 (2009).
Zhang, G. Q., Wei, H., Lu, J., Wong, P. & Shim, W. Identification and characterization of calcium sparks in cardiomyocytes derived from human induced pluripotent stem cells. PLoS ONE 8, e55266 (2013).
Rodriguez, M. L. et al. Measuring the contractile forces of human induced pluripotent stem cell-derived cardiomyocytes with arrays of microposts. J. Biomech. Eng. 136, 0510051–0510010 (2014).
Li, S., Chen, G. & Li, R. A. Calcium signalling of human pluripotent stem cell-derived cardiomyocytes. J. Physiol. 591, 5279–5290 (2013).
Li, S., Cheng, H., Tomaselli, G. F. & Li, R. A. Mechanistic basis of excitation−contraction coupling in human pluripotent stem cell-derived ventricular cardiomyocytes revealed by Ca2+ spark characteristics: direct evidence of functional Ca2+-induced-Ca2+-release. Heart Rhythm 11, 133–140 (2013).
Blazeski, A. et al. Electrophysiological and contractile function of cardiomyocytes derived from human embryonic stem cells. Prog. Biophys. Mol. Biol. 110, 178–195 (2012).
Sedan, O. K. & Binah, O. in Regenerating the Heart (eds Cohen, I. S. & Gaudette, G. R.) 37–52 (Humana Press, 2011).
Robertson, C., Tran, D. D. & George, S. C. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells 31, 829–837 (2013).
Binah, O. et al. Functional and developmental properties of human embryonic stem cell-derived cardiomyocytes. J. Electrocardiol. 40, S192–S196 (2007).
Germanguz, I. et al. Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells. J. Cell. Mol. Med. 15, 38–51 (2011).
Nunes, S. S. et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat. Methods 10, 781–787 (2013).
Rao, C. et al. The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials 34, 2399–2411 (2013).
Yang, X., Pabon, L. & Murry, C. E. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 114, 511–523 (2014).
Xi, J. et al. Comparison of contractile behavior of native murine ventricular tissue and cardiomyocytes derived from embryonic or induced pluripotent stem cells. FASEB J. 24, 2739–2751 (2010).
Pillekamp, F. et al. Contractile properties of early human embryonic stem cell-derived cardiomyocytes: beta-adrenergic stimulation induces positive chronotropy and lusitropy but not inotropy. Stem Cells Dev. 10, 2111–2121 (2012).
Montaigne, D., Hurt, C. & Neviere, R. Mitochondria death/survival signaling pathways in cardiotoxicity induced by anthracyclines and anticancer-targeted therapies. Biochem. Res. Int. 951539 (2012).
Mellor, H. R., Bell, A. R., Valentin, J. P. & Roberts, R. R. Cardiotoxicity associated with targeting kinase pathways in cancer. Toxicol. Sci. 120, 14–32 (2011).
O'Brien, P. J. et al. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch. Toxicol. 89, 580–604 (2006).
Towne, D. L. et al. Development of a high-content screening assay panel to accelerate mechanism of action studies for oncology research. J. Biomol. Screen. 17, 1005–1017 (2012).
Carlson, C. et al. Phenotypic screening with human iPS cell-derived cardiomyocytes: HTS-compatible assays for interrogating cardiac hypertrophy. J. Biomol. Screen. 18, 1203–1211 (2013).
Persson, M., Loye, A. F., Mow, T. & Hornberg, J. J. A high content screening assay to predicte human drug-induced liver injury during drug discovery. J. Pharmacol. Toxicol. Methods 68, 302–313 (2013).
Pointon, A., Abi-Gerges, N., Cross, M. J. & Sidaway, J. E. Phenotypic profiling of structural cardiotoxins in vitro reveals dependency on multiple mechanisms of toxicity. Toxicol. Sci. 132, 317–326 (2013).
Mioulane, M., Foldes, G., Ali, N. N., Schneider, M. D. & Harding, S. E. Development of high content imaging methods for cell death detection in human pluripotent stem cell-derived cardiomyocytes. J. Cardiovasc. Transl. Res. 5, 593–604 (2012).
Doherty, K. R. et al. Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes. Toxicol. Appl. Pharmacol. 272, 245 (2013).
Li, J. et al. Profiling of nutrient transporter expression in human stem cell-derived cardiomyocytes exposed to tyrosine kinase inhibitor anticancer drugs using RBD ligands. J. Biomol. Screen. 19, 1185–1192 (2014).
Singh, S., Carpenter, A. E. & Genovesio, A. Increasing the content of high-content screening: an overview. J. Biomol. Screen. 19, 640–650 (2014).
Guo, L. et al. Refining the human iPSC-cardiomyocyte arrhythmic risk (hCAR) assessment model. Toxicol. Sci. 136, 581–594 (2013).
Scannell, J. W., Blanckley, A., Boldon, H. & Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 11, 193–200 (2012).
Swinney, D. C. & Anthony, J. How were new medicines discovered? Nat. Rev. Drug Discov. 10, 507–519 (2011).
Lee, J. A. & Berg, E. L. Neoclassic drug discovery: the case for lead generation using phenotypic and functional approaches. J. Biomol. Screen. 10, 1143–1155 (2013).
Hardy, M. E., Lawrence, C. L., Standen, N. B. & Rodrigo, G. C. Can optical recordings of membrane potential be used to screen for drug-induced action potential prolongation in single cardiac myocytes? J. Pharmacol. Toxicol. Methods 54, 173–182 (2006).
Kaestner, L. & Lipp, P. Screening action potentials: the power of light. Front. Pharmacol. 2, 42 (2011).
Meyer, T., Boven, K. H., Günther, E. & Fejtl, M. Micro-electrode arrays in cardiac safety pharmacology: a novel tool to study QT interval prolongation. Drug Saf. 27, 763–772 (2004).
Braam, S. R. et al. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 4, 107–116 (2010).
Grilo, L. S., Carrupt, P. A. & Abriel, H. Stereoselective inhibition of the hERG1 potassium channel. Front. Pharmacol. 1, 137 (2010).
Acknowledgements
The authors wish to thank N. Thomas for helpful discussions and insights regarding high-content screening approaches with human stem cell-derived cardiomyocytes and for reviewing the manuscript. The authors also acknowledge multiple discussions with numerous individuals regarding new in vitro approaches that led to the development of the Comprehensive in vitro Proarrhythmia Assay (CiPA) and J. Green and J. Sutherland for their support with in silico reconstructions. AbbVie participated in the review and approval of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
P.T.S. is a cardiac safety consultant (a member of the Data Safety and Monitoring Board, a member of the cardiovascular end-point committee, a consultant or an advisory board member) to Chemo, Milestone Pharmaceuticals, Medtronic, Cellceutix, Halozyme, NeuroVia, Trevi, S. K. Science, Viamet, Shin Nippon Biomedical Laboratories (SNBL), Biomedical Systems, iCardiac, Dart, Cancer Prevention, AbbVie, Heart Metabolics, Acadia, MyoKardia, Biogen, Theravance, Charles River, Acesion, Vivus and NDA Partners. Finally, P.T.S. is also a member of the Board of Directors of Anthera, Inc. He has no financial stake in any of the technologies discussed in the manuscript.
Rights and permissions
About this article
Cite this article
Gintant, G., Sager, P. & Stockbridge, N. Evolution of strategies to improve preclinical cardiac safety testing. Nat Rev Drug Discov 15, 457–471 (2016). https://doi.org/10.1038/nrd.2015.34
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2015.34
This article is cited by
-
The evolving role of investigative toxicology in the pharmaceutical industry
Nature Reviews Drug Discovery (2023)
-
The atypic antipsychotic clozapine inhibits multiple cardiac ion channels
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
The Advantages, Challenges, and Future of Human-Induced Pluripotent Stem Cell Lines in Type 2 Long QT Syndrome
Journal of Cardiovascular Translational Research (2023)
-
Tyrosine Kinase Inhibitor Si409 Has In Vitro and In Vivo Anti-Tumor Activity Against Diffuse Large B-Cell Lymphoma
Pharmaceutical Chemistry Journal (2023)
-
Uncertainty assessment of proarrhythmia predictions derived from multi-level in silico models
Archives of Toxicology (2023)