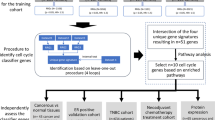
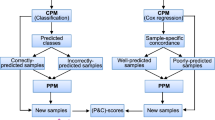
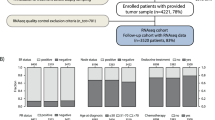
Key Points
-
Breast cancer is not a single, homogenous disease, but a heterogeneous group of different disease subtypes, each with its own biological and clinical characteristics
-
Methods to enhance the identification of individuals with high-risk disease, who would benefit from more-intensive treatment are an area of expanding research interest
-
The development of multigene assays has led to improvements in predicting the risk of recurrence in patients with early stage oestrogen receptor (ER)-positive, HER2-negative breast cancer, compared with use of standard prognostic criteria
-
Several multigene assays, including Oncotype DX, Prosigna, and MammaPrint have emerged as well-studied technologies and have already been incorporated into clinical practice, to some extent
-
In 2016, the ASCO Breast Cancer Guidelines Advisory Group and Clinical Practice Guidelines Committee published updated guidelines for the use of biomarkers, including gene-expression assays, to help guide the use of adjuvant systemic therapy
-
Several large, randomized trials are currently ongoing and aim to prospectively evaluate and further validate the performance of these assays, in order to provide the highest level of evidence of clinical utility
Abstract
Breast cancer is a heterogeneous disease, with different subtypes having a distinct biological, molecular, and clinical course. Assessments of standard clinical and pathological features have traditionally been used to determine the use of adjuvant systemic therapy in patients with early stage breast cancer; however, the ability to identify those who will benefit from adjuvant chemotherapy remains a challenge, leading to the overtreatment of some patients. Advances in molecular medicine have substantially improved the accuracy of gene-expression profiling of breast tumours, resulting in improvements in the ability to predict a patient's risk of breast cancer recurrence and likely response to endocrine therapy and/or chemotherapy. These genomic assays, several of which are commercially available, have aided physicians in tailoring treatment decisions for patients at the individual level. Herein, we describe the available data on the clinical validity of the most widely available assays in patients with early stage breast cancer, with a focus on the development, validation, and clinical application of these assays, in addition to the anticipated outcomes of ongoing prospective trials. We also review data from comparative studies of these assays and from cost-effectiveness analyses relating to their clinical use.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 66, 7–30 (2016).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Gruvberger, S. et al. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 61, 5979–5984 (2001).
Sorlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA 98, 10869–10874 (2001).
Esteva, F. J., Sahin, A. A., Cristofanilli, M., Arun, B. & Hortobagyi, G. N. Molecular prognostic factors for breast cancer metastasis and survival. Semin. Radiat. Oncol. 12, 319–328 (2002).
Hammond, M. E. et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 28, 2784–2795 (2010).
Wolff, A. C. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 31, 3997–4013 (2013).
Esteva, F. J. Monoclonal antibodies, small molecules, and vaccines in the treatment of breast cancer. Oncologist 9, 4–9 (2004).
Morrow, P. K., Zambrana, F. & Esteva, F. J. Recent advances in systemic therapy: advances in systemic therapy for HER2-positive metastatic breast cancer. Breast Cancer Res. 11, 207 (2009).
Fornier, M., Esteva, F. J. & Seidman, A. D. Trastuzumab in combination with chemotherapy for the treatment of metastatic breast cancer. Semin. Oncol. 27, 38–45 (2000).
Burstein, H. J. et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J. Clin. Oncol. 32, 2255–2269 (2014).
Ravdin, P. M. et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J. Clin. Oncol. 19, 980–991 (2001).
Yersal, O. & Barutca, S. Biological subtypes of breast cancer: prognostic and therapeutic implications. World J. Clin. Oncol. 5, 412–424 (2014).
Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365, 1687–1717 (2005).
Early Breast Cancer Trialists' Collaborative Group et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378, 771–784 (2011).
Early Breast Cancer Trialists' Collaborative Group et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379, 432–444 (2012).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Cheang, M. C. et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J. Natl Cancer Inst. 101, 736–750 (2009).
Sotiriou, C. et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl Acad. Sci. USA 100, 10393–10398 (2003).
Rouzier, R. et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 11, 5678–5685 (2005).
Prat, A. et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 12, R68 (2010).
Harris, L. N. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 34, 1134–1150 (2016).
Teutsch, S. M. et al. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) initiative: methods of the EGAPP Working Group. Genet. Med. 11, 3–14 (2009).
Botkin, J. R. et al. Outcomes of interest in evidence-based evaluations of genetic tests. Genet. Med. 12, 228–235 (2010).
Paik, S. et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 351, 2817–2826 (2004).
Esteban, J. et al. Tumor gene expression and prognosis in breast cancer: multi-gene RT-PCR assay of paraffin-embedded tissue [abstract]. Proc. Am. Soc. Clin. Oncol. 22, 850 (2003).
Cobleigh, M. A. et al. Tumor gene expression predicts distant disease- free survival (DDFS) in breast cancer patients with 10 or more positive nodes: high throughout RT-PCR assay of paraffin-embedded tumor tissues [abstract]. Proc. Am. Soc. Clin. Oncol. 22, 850 (2003).
Paik, S. et al. Multi-gene RT-PCR assay for predicting recurrence in node negative breast cancer patients — NSABP studies B-20 and B-14. Breast Cancer Res. Treat. 82, A16 (2003).
Esteva, F. J. et al. Prognostic role of a multigene reverse transcriptase-PCR assay in patients with node-negative breast cancer not receiving adjuvant systemic therapy. Clin. Cancer Res. 11, 3315–3319 (2005).
Paik, S. et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 24, 3726–3734 (2006).
Goldstein, L. J. et al. Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American Breast Cancer Intergroup Trial E 2197. J. Clin. Oncol. 26, 4092–4099 (2008).
Goldstein, L. J. et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J. Clin. Oncol. 26, 4063–4071 (2008).
Dowsett, M. et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J. Clin. Oncol. 28, 1829–1834 (2010).
Hayes, D. F. Biomarker validation and testing. Mol. Oncol. 9, 960–966 (2015).
Sparano, J. A. TAILORx: trial assigning individualized options for treatment (Rx). Clin. Breast Cancer 7, 347–350 (2006).
Sparano, J. A. et al. Prospective validation of a 21-gene expression assay in breast cancer. N. Engl. J. Med. 373, 2005–2014 (2015).
Parker, J. S. et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 27, 1160–1167 (2009).
Brumbaugh, C. D., Kim, H. J., Giovacchini, M. & Pourmand, N. NanoStriDE: normalization and differential expression analysis of NanoString nCounter data. BMC Bioinformatics 12, 479 (2011).
Hess, K. R. et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J. Clin. Oncol. 24, 4236–4244 (2006).
Esteva, F. J. et al. Expression of erbB/HER receptors, heregulin and P38 in primary breast cancer using quantitative immunohistochemistry. Pathol. Oncol. Res. 7, 171–177 (2001).
Nielsen, T. O. et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin. Cancer Res. 16, 5222–5232 (2010).
Gnant, M. et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann. Oncol. 25, 339–345 (2014).
van 't Veer, L. J. et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530–536 (2002).
van de Vijver, M. J. et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347, 1999–2009 (2002).
Drukker, C. A. et al. Long-term impact of the 70-gene signature on breast cancer outcome. Breast Cancer Res. Treat. 143, 587–592 (2014).
Buyse, M. et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J. Natl Cancer Inst. 98, 1183–1192 (2006).
Drukker, C. A. et al. A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int. J. Cancer 133, 929–936 (2013).
Kwaliteitsinstituut voor de Gezondheidszorg CBO VvlK. Adjuvante Systemische Therapie voor het Operabel Mammacarcinoom. Richtlijn Behandeling van het Mammacarcinoom [Dutch]. 46–70 (2004).
Bueno-de-Mesquita, J. M., Sonke, G. S., van de Vijver, M. J. & Linn, S. C. Additional value and potential use of the 70-gene prognosis signature in node-negative breast cancer in daily clinical practice. Ann. Oncol. 22, 2021–2030 (2011).
Wittner, B. S. et al. Analysis of the MammaPrint breast cancer assay in a predominantly postmenopausal cohort. Clin. Cancer Res. 14, 2988–2993 (2008).
Drukker, C. A. et al. Gene expression profiling to predict the risk of locoregional recurrence in breast cancer: a pooled analysis. Breast Cancer Res. Treat. 148, 599–613 (2014).
Mook, S. et al. The 70-gene prognosis-signature predicts disease outcome in breast cancer patients with 1–3 positive lymph nodes in an independent validation study. Breast Cancer Res. Treat. 116, 295–302 (2009).
Mook, S. et al. The 70-gene prognosis signature predicts early metastasis in breast cancer patients between 55 and 70 years of age. Ann. Oncol. 21, 717–722 (2010).
Saghatchian, M. et al. Additional prognostic value of the 70-gene signature (MammaPrint®) among breast cancer patients with 4–9 positive lymph nodes. Breast 22, 682–690 (2013).
Sapino, A. et al. MammaPrint molecular diagnostics on formalin-fixed, paraffin-embedded tissue. J. Mol. Diagn. 16, 190–197 (2014).
Mittempergher, L. et al. Gene expression profiles from formalin fixed paraffin embedded breast cancer tissue are largely comparable to fresh frozen matched tissue. PLoS ONE 6, e17163 (2011).
Cardoso, F. et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N. Engl. J. Med. 375, 717–729 (2016).
Ma, X. J. et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell 5, 607–616 (2004).
Ma, X. J. et al. A five-gene molecular grade index and HOXB13:IL17BR are complementary prognostic factors in early stage breast cancer. Clin. Cancer Res. 14, 2601–2608 (2008).
Jerevall, P. L. et al. Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br. J. Cancer 104, 1762–1769 (2011).
Habel, L. A. et al. HOXB13:IL17BR and molecular grade index and risk of breast cancer death among patients with lymph node-negative invasive disease. Breast Cancer Res. 15, R24 (2013).
Goss, P. E. et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N. Engl. J. Med. 349, 1793–1802 (2003).
National Comprehensive Cancer Network. NCCN clinical practice guidelines, breast cancer, 2016. Version 2.2016 (NCCN, 2016).
Sgroi, D. C. et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J. Natl Cancer Inst. 105, 1036–1042 (2013).
Filipits, M. et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin. Cancer Res. 17, 6012–6020 (2011).
Kronenwett, R. et al. Decentral gene expression analysis: analytical validation of the Endopredict genomic multianalyte breast cancer prognosis test. BMC Cancer 12, 456 (2012).
Dubsky, P. et al. EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann. Oncol. 24, 640–647 (2013).
Dubsky, P. et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br. J. Cancer 109, 2959–2964 (2013).
Schmid, M. et al. Randomized trial of tamoxifen versus tamoxifen plus aminoglutethimide as adjuvant treatment in postmenopausal breast cancer patients with hormone receptor-positive disease: Austrian breast and colorectal cancer study group trial 6. J. Clin. Oncol. 21, 984–990 (2003).
Jakesz, R. et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366, 455–462 (2005).
Martin, M. et al. Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2- breast cancer patients: results from the GEICAM 9906 trial. Breast Cancer Res. 16, R38 (2014).
Cuzick, J. et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J. Clin. Oncol. 29, 4273–4278 (2011).
Polley, M. Y. et al. An international Ki67 reproducibility study. J. Natl Cancer Inst. 105, 1897–1906 (2013).
Sotiriou, C. et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J. Natl Cancer Inst. 98, 262–272 (2006).
Loi, S. et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J. Clin. Oncol. 25, 1239–1246 (2007).
Liedtke, C. et al. Genomic grade index is associated with response to chemotherapy in patients with breast cancer. J. Clin. Oncol. 27, 3185–3191 (2009).
Fan, C. et al. Concordance among gene-expression-based predictors for breast cancer. N. Engl. J. Med. 355, 560–569 (2006).
Sorlie, T. et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl Acad. Sci. USA 100, 8418–8423 (2003).
Chang, H. Y. et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc. Natl Acad. Sci. USA 102, 3738–3743 (2005).
Chang, H. Y. et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2, E7 (2004).
Wirapati, P. et al. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 10, R65 (2008).
Dowsett, M. et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J. Clin. Oncol. 31, 2783–2790 (2013).
Kelly, C. M. et al. Utility of oncotype DX risk estimates in clinically intermediate risk hormone receptor-positive, HER2-normal, grade II, lymph node-negative breast cancers. Cancer 116, 5161–5167 (2010).
Kelly, C. M. et al. Agreement in risk prediction between the 21-gene recurrence score assay (Oncotype DX®) and the PAM50 Breast Cancer Intrinsic Classifier in early-stage estrogen receptor-positive breast cancer. Oncologist 17, 492–498 (2012).
Sgroi, D. C. et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 14, 1067–1076 (2013).
Ignatiadis, M. et al. The Genomic Grade assay compared with Ki67 to determine risk of distant breast cancer recurrence. JAMA Oncol. 2, 217–224 (2016).
Breast International Group (BIG) 1–98 Collaborative Group et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N. Engl. J. Med. 353, 2747–2757 (2005).
Bartlett, J. M. et al. Comparing breast cancer multiparameter tests in the OPTIMA prelim trial: no test is more equal than the others. J. Natl Cancer Inst. 108, djw050 (2016).
Sestack, I. et al. Comprehensive comparison of prognostic signatures for breast cancer in TransATAC [abstract]. 2016 San Antonio Breast Cancer Symposium (SABCS). Abstr. S6-05 (2016).
Bajdik, I. A. et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J. Clin. Oncol. 23, 2716–2725 (2005).
Retel, V. P. et al. Prospective cost-effectiveness analysis of genomic profiling in breast cancer. Eur. J. Cancer 49, 3773–3779 (2013).
Lamond, N. W., Skedgel, C., Rayson, D., Lethbridge, L. & Younis, T. Cost-utility of the 21-gene recurrence score assay in node-negative and node-positive breast cancer. Breast Cancer Res. Treat. 133, 1115–1123 (2012).
Lyman, G. H., Cosler, L. E., Kuderer, N. M. & Hornberger, J. Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies. Cancer 109, 1011–1018 (2007).
Chen, E., Tong, K. B. & Malin, J. L. Cost-effectiveness of 70-gene MammaPrint signature in node-negative breast cancer. Am. J. Manag. Care 16, e333–e342 (2010).
Retel, V. P. et al. Cost-effectiveness of the 70-gene signature versus St. Gallen guidelines and Adjuvant Online for early breast cancer. Eur. J. Cancer 46, 1382–1391 (2010).
Ward, S. et al. Gene expression profiling and expanded immunohistochemistry tests to guide the use of adjuvant chemotherapy in breast cancer management: a systematic review and cost-effectiveness analysis. Health Technol. Assess. 17, 1–302 (2013).
Rouzier, R. et al. Multigene assays and molecular markers in breast cancer: systematic review of health economic analyses. Breast Cancer Res. Treat. 139, 621–637 (2013).
Yang, M., Rajan, S. & Issa, A. M. Cost effectiveness of gene expression profiling for early stage breast cancer: a decision-analytic model. Cancer 118, 5163–5170 (2012).
Griffin, A. M. et al. On the receiving end. V: patient perceptions of the side effects of cancer chemotherapy in 1993. Ann. Oncol. 7, 189–195 (1996).
Azim, H. A. Jr et al. Utility of prognostic genomic tests in breast cancer practice: the IMPAKT 2012 Working Group consensus statement. Ann. Oncol. 24, 647–654 (2013).
Harris, L. et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J. Clin. Oncol. 25, 5287–5312 (2007).
Harbeck, N. et al. Ten-year analysis of the prospective multicentre Chemo-N0 trial validates American Society of Clinical Oncology (ASCO)-recommended biomarkers uPA and PAI-1 for therapy decision making in node-negative breast cancer patients. Eur. J. Cancer 49, 1825–1835 (2013).
Ross, D. T. et al. Chemosensitivity and stratification by a five monoclonal antibody immunohistochemistry test in the NSABP B14 and B20 trials. Clin. Cancer Res. 14, 6602–6609 (2008).
Desmedt, C. et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin. Cancer Res. 14, 5158–5165 (2008).
Loi, S. et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J. Clin. Oncol. 31, 860–867 (2013).
Loi, S. et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann. Oncol. 25, 1544–1550 (2014).
Albain, K. S. et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet 374, 2055–2063 (2009).
DeSantis, C., Ma, J., Bryan, L. & Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 64, 52–62 (2014).
Lewis, J. H. et al. Participation of patients 65 years of age or older in cancer clinical trials. J. Clin. Oncol. 21, 1383–1389 (2003).
Wildiers, H. et al. The EORTC Cancer in the Elderly Task Force, a Protostar for EORTC's future. Eur. J. Cancer 10, 34–38 (2012).
Acknowledgements
The authors would like to thank Mr. Gordon Cook of the NYU Langone Medical Center for graphical design assistance with the manuscript figures.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Supplementary information
Supplementary information S1 (figure)
Current prospective clinical trials incorporating gene-expression assays. (PDF 571 kb)
Rights and permissions
About this article
Cite this article
Kwa, M., Makris, A. & Esteva, F. Clinical utility of gene-expression signatures in early stage breast cancer. Nat Rev Clin Oncol 14, 595–610 (2017). https://doi.org/10.1038/nrclinonc.2017.74
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2017.74
This article is cited by
-
Caveolin-1 gene expression provides additional prognostic information combined with PAM50 risk of recurrence (ROR) score in breast cancer
Scientific Reports (2024)
-
Real-world use of multigene signatures in early breast cancer: differences to clinical trials
Breast Cancer Research and Treatment (2024)
-
Looking ahead in early-phase trial design to improve the drug development process: examples in oncology
BMC Medical Research Methodology (2023)
-
The heterogeneity of tumour immune microenvironment revealing the CRABP2/CD69 signature discriminates distinct clinical outcomes in breast cancer
British Journal of Cancer (2023)
-
Caveolin-1 genotypes as predictor for locoregional recurrence and contralateral disease in breast cancer
Breast Cancer Research and Treatment (2023)