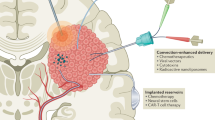
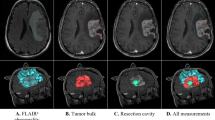
Key Points
-
Surgery remains the mainstay treatment of gliomas, with maximal resection of the tumour being central to achieving long-term disease control; growing evidence supports efforts to undertake more-extensive 'supratotal' resection
-
The real clinical benefit of glioma surgery depends, however, on the balance between the extent of cytoreduction and neurological morbidity; novel surgical techniques and technologies can be leveraged to improve both of these determinants of patient outcomes
-
Advanced intraoperative imaging methods (such as intraoperative neuronavigation, MRI, and ultrasonography), fluorescence-based tumour biomarkers, and real-time mutational analyses can be exploited to maximize tumour resection
-
In parallel, the risk of perioperative morbidity can be minimized through the combined use of corticospinal tract imaging (MRI-based diffusion tensor imaging tractography and transcranial magnetic stimulation), stimulation mapping, and/or somatosensory-evoked potential techniques
-
Together, these technological advances and modern principles of neurosurgical oncology have dramatically altered the approach to the treatment of patients with glioma and have enabled improvements in clinical outcomes
Abstract
Surgical resection remains the mainstay of treatment for patients with glioma of any grade. Maximal resection of the tumour is central to achieving long-term disease control; however, the relationship between the extent of glioma resection and actual clinical benefit for the patient is predicated on the balance between cytoreduction and neurological morbidity. For the neurosurgical oncologist, the clinical rationale for undertaking increasingly extensive resections has gained traction. In parallel, novel surgical techniques and technologies have been developed that help improve patient outcomes. During the past decade, neurosurgeons have leveraged advanced intraoperative imaging methods, fluorescence-based tumour biomarkers, and real-time mutational analyses to maximize the extent of tumour resection. In addition, approaches to minimizing the risk of perioperative morbidity continue to be improved through the combined use of stimulation-mapping techniques, corticospinal tract imaging, and stereotactic thermal ablation. Taken together, these modern principles of neurosurgical oncology bear little resemblance to historical therapeutic strategies for patients with glioma and have dramatically altered the approach to the treatment of patients with these brain tumours. Herein, we outline the state of the art in surgical oncology for gliomas.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 18 (Suppl. 5), v1–v75 (2016).
Gilbert, M. R. et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 370, 699–708 (2014).
Sanai, N., Chang, S. & Berger, M. S. Low-grade gliomas in adults. J. Neurosurg. 115, 948–965 (2011).
Sanai, N. & Berger, M. S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 62, 753–764 (2008).
Reifenberger, G., Wirsching, H. G., Knobbe-Thomsen, C. B. & Weller, M. Advances in the molecular genetics of gliomas — implications for classification and therapy. Nat. Rev. Clin. Oncol. 14, 434–452 (2017).
Guthrie, B. L. & Laws, E. R. Jr. Supratentorial low-grade gliomas. Neurosurg. Clin. N. Am. 1, 37–48 (1990).
Bauman, G. et al. Pretreatment factors predict overall survival for patients with low-grade glioma: a recursive partitioning analysis. Int. J. Radiat. Oncol. Biol. Phys. 45, 923–929 (1999).
Janny, P. et al. Low grade supratentorial astrocytomas. Management and prognostic factors. Cancer 73, 1937–1945 (1994).
Karim, A. B. et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int. J. Radiat. Oncol. Biol. Phys. 36, 549–556 (1996).
Laws, E. R. et al. The neurosurgical management of low-grade astrocytoma. Clin. Neurosurg. 33, 575–588 (1986).
Lote, K. et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J. Clin. Oncol. 15, 3129–3140 (1997).
Piepmeier, J. et al. Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgery 38, 872–878 (1996).
Pignatti, F. et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J. Clin. Oncol. 20, 2076–2084 (2002).
Shaw, E. et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J. Clin. Oncol. 20, 2267–2276 (2002).
van den Bent, M. J. et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366, 985–990 (2005).
Pallud, J. et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain 137, 449–462 (2014).
Ramakrishna, R., Hebb, A., Barber, J., Rostomily, R. & Silbergeld, D. Outcomes in reoperated low-grade gliomas. Neurosurgery 77, 175–184 (2015).
Smith, J. S. et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J. Clin. Oncol. 26, 1338–1345 (2008).
Ius, T. et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J. Neurosurg. 117, 1039–1052 (2012).
Capelle, L. et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J. Neurosurg. 118, 1157–1168 (2013).
Xu, D. S. et al. An extent of resection threshold for seizure freedom in patients with low-grade gliomas. J. Neurosurg. 26, 1–7 (2017).
Yordanova, Y. N. & Duffau, H. Supratotal resection of diffuse gliomas — an overview of its multifaceted implications. Neurochirurgie 63, 243–249 (2017).
Jakola, A. S. et al. Comparison of a strategy favoring early surgical resection versus a strategy favoring watchful waiting in low-grade gliomas. JAMA 308, 1881–1888 (2012).
Stummer, W. et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 7, 392–401 (2006).
Stummer, W. et al. Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clinical article. J. Neurosurg. 114, 613–623 (2011).
Stummer, W. et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62, 564–576 (2008).
Brown, T. J. et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2, 1460–1469 (2016).
Hrabalek, L. et al. Resection versus biopsy of glioblastomas in eloquent brain areas. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 159, 150–155 (2015).
Kreth, F. W. et al. The role of tumor resection in the treatment of glioblastoma multiforme in adults. Cancer 86, 2117–2123 (1999).
Li, Y. M., Suki, D., Hess, K. & Sawaya, R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J. Neurosurg. 124, 977–988 (2016).
Sanai, N., Polley, M. Y., McDermott, M. W., Parsa, A. T. & Berger, M. S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 115, 3–8 (2011).
Lacroix, M. et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J. Neurosurg. 95, 190–198 (2001).
Pessina, F. et al. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? A single institution retrospective experience. J. Neurooncol. 135, 129–139 (2017).
Chaichana, K. L. et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 16, 113–122 (2014).
Oppenlander, M. E. et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J. Neurosurg. 120, 846–853 (2014).
Warnke, P. C. Stereotactic volumetric resection of gliomas. Acta Neurochir. Suppl. 88, 5–8 (2003).
Krishnan, R. et al. Functional magnetic resonance imaging-integrated neuronavigation: correlation between lesion-to-motor cortex distance and outcome. Neurosurgery 55, 904–914 (2004).
Wu, J. S. et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery 61, 935–948 (2007).
Reithmeier, T., Krammer, M., Gumprecht, H., Gerstner, W. & Lumenta, C. B. Neuronavigation combined with electrophysiological monitoring for surgery of lesions in eloquent brain areas in 42 cases: a retrospective comparison of the neurological outcome and the quality of resection with a control group with similar lesions. Minim. Invasive Neurosurg. 46, 65–71 (2003).
Kurimoto, M. et al. Impact of neuronavigation and image-guided extensive resection for adult patients with supratentorial malignant astrocytomas: a single-institution retrospective study. Minim. Invasive Neurosurg. 47, 278–283 (2004).
Willems, P. W., Taphoorn, M. J., Burger, H., Berkelbach van der Sprenkel, J. W. & Tulleken, C. A. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J. Neurosurgery 104, 360–368 (2006).
Unsgaard, G. et al. Ability of navigated 3D ultrasound to delineate gliomas and metastases — comparison of image interpretations with histopathology. Acta Neurochir. 147, 1259–1269 (2005).
Coenen, V. A. et al. Sequential visualization of brain and fiber tract deformation during intracranial surgery with three-dimensional ultrasound: an approach to evaluate the effect of brain shift. Neurosurgery 56, 133–141 (2005).
Reinacher, P. C. & van Velthoven, V. Intraoperative ultrasound imaging: practical applicability as a real-time navigation system. Acta Neurochir. Suppl. 85, 89–93 (2003).
Nikas, D. C. et al. Coregistered intraoperative ultrasonography in resection of malignant glioma. Neurosurg. Focus 14, e6 (2003).
Senft, C., Seifert, V., Hermann, E., Franz, K. & Gasser, T. Usefulness of intraoperative ultra low-field magnetic resonance imaging in glioma surgery. Neurosurgery 63, 257–266 (2008).
Nimsky, C., Fujita, A., Ganslandt, O., Von Keller, B. & Fahlbusch, R. Volumetric assessment of glioma removal by intraoperative high-field magnetic resonance imaging. Neurosurgery 55, 358–370 (2004).
Hall, W. A., Liu, H., Maxwell, R. E. & Truwit, C. L. Influence of 1.5-Tesla intraoperative MR imaging on surgical decision making. Acta Neurochir. Suppl. 85, 29–37 (2003).
Hirschberg, H., Samset, E., Hol, P. K., Tillung, T. & Lote, K. Impact of intraoperative MRI on the surgical results for high-grade gliomas. Minim. Invasive Neurosurg. 48, 77–84 (2005).
Senft, C., Bink, A., Heckelmann, M., Gasser, T. & Seifert, V. Glioma extent of resection and ultra-low-field iMRI: interim analysis of a prospective randomized trial. Acta Neurochir. Suppl. 109, 49–53 (2011).
Hatiboglu, M. A. et al. Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis. Neurosurgery 64, 1073–1081 (2009).
Pamir, M. N. et al. First intraoperative, shared-resource, ultrahigh-field 3-Tesla magnetic resonance imaging system and its application in low-grade glioma resection. J. Neurosurg. 112, 57–69 (2010).
Black, P. M. et al. Craniotomy for tumor treatment in an intraoperative magnetic resonance imaging unit. Neurosurgery 45, 423–431 (1999).
Claus, E. B. et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer 103, 1227–1233 (2005).
Barone, D. G., Lawrie, T. A. & Hart, M. G. Image guided surgery for the resection of brain tumours. Cochrane Database Syst. Rev. 1, CD009685 (2014).
Senft, C. et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 12, 997–1003 (2011).
Nimsky, C., Ganslandt, O., Tomandl, B., Buchfelder, M. & Fahlbusch, R. Low-field magnetic resonance imaging for intraoperative use in neurosurgery: a 5-year experience. Eur. Radiol. 12, 2690–2703 (2002).
Muragaki, Y. et al. Information-guided surgical management of gliomas using low-field-strength intraoperative MRI. Acta Neurochir. Suppl. 109, 67–72 (2011).
Kubben, P. L. et al. Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol. 12, 1062–1070 (2011).
Schneider, J. P. et al. Intraoperative MRI to guide the resection of primary supratentorial glioblastoma multiforme — a quantitative radiological analysis. Neuroradiology 47, 489–500 (2005).
Chandler, W. F., Knake, J. E., McGillicuddy, J. E., Lillehei, K. O. & Silver, T. M. Intraoperative use of real-time ultrasonography in neurosurgery. J. Neurosurg. 57, 157–163 (1982).
Prada, F. et al. Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery 74, 542–552 (2014).
Gerganov, V. M. et al. Reliability of intraoperative high-resolution 2D ultrasound as an alternative to high-field strength MR imaging for tumor resection control: a prospective comparative study. J. Neurosurg. 111, 512–519 (2009).
Coburger, J. et al. Navigated high frequency ultrasound: description of technique and clinical comparison with conventional intracranial ultrasound. World Neurosurg. 82, 366–375 (2014).
Solheim, O., Selbekk, T., Jakola, A. S. & Unsgard, G. Ultrasound-guided operations in unselected high-grade gliomas — overall results, impact of image quality and patient selection. Acta Neurochir. 152, 1873–1886 (2010).
Stummer, W. et al. Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir. 140, 995–1000 (1998).
Duffner, F. et al. Specific intensity imaging for glioblastoma and neural cell cultures with 5-aminolevulinic acid-derived protoporphyrin IX. J. Neurooncol. 71, 107–111 (2005).
Stummer, W., Reulen, H. J., Novotny, A., Stepp, H. & Tonn, J. C. Fluorescence-guided resections of malignant gliomas — an overview. Acta Neurochir. Suppl. 88, 9–12 (2003).
Ishihara, R. et al. Quantitative spectroscopic analysis of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence intensity in diffusely infiltrating astrocytomas. Neurol. Med. Chir. 47, 53–57 (2007).
Zhang, R. R. et al. Beyond the margins: real-time detection of cancer using targeted fluorophores. Nat. Rev. Clin. Oncol. 14, 347–364 (2017).
Floeth, F. W. et al. Comparison of 18F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur. J. Nucl. Med. Mol. Imag. 38, 731–741 (2010).
Sanai, N. et al. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J. Neurosurg. 115, 740–748 (2011).
Sanai, N. et al. Intraoperative confocal microscopy for brain tumors: a feasibility analysis in humans. Neurosurgery 68, 282–290 (2011).
Sankar, T. et al. Miniaturized handheld confocal microscopy for neurosurgery: results in an experimental glioblastoma model. Neurosurgery 66, 410–417 (2010).
Eberlin, L. S. et al. Ambient mass spectrometry for the intraoperative molecular diagnosis of human brain tumors. Proc. Natl Acad. Sci. USA 110, 1611–1616 (2013).
Gholami, B., Agar, N. Y., Jolesz, F. A., Haddad, W. M. & Tannenbaum, A. R. A compressive sensing approach for glioma margin delineation using mass spectrometry. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011, 5682–5685 (2011).
Shankar, G. M. et al. Rapid intraoperative molecular characterization of glioma. JAMA Oncol. 1, 662–667 (2015).
Kanamori, M. et al. Rapid and sensitive intraoperative detection of mutations in the isocitrate dehydrogenase 1 and 2 genes during surgery for glioma. J. Neurosurg. 120, 1288–1297 (2014).
Kim, C. S. et al. Characterization of invading glioma cells using molecular analysis of leading-edge tissue. J. Kor. Neurosurg. Soc. 50, 157–165 (2011).
Alexiou, G. A. et al. Fast cell cycle analysis for intraoperative characterization of brain tumor margins and malignancy. J. Clin. Neurosci. 22, 129–132 (2015).
Rennert, R. C., Santiago-Dieppa, D. R., Figueroa, J., Sanai, N. & Carter, B. S. Future directions of operative neuro-oncology. J. Neurooncol. 130, 377–382 (2016).
Beiko, J. et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 16, 81–91 (2014).
Mazor, T., Pankov, A., Song, J. S. & Costello, J. F. Intratumoral heterogeneity of the epigenome. Cancer Cell 29, 440–451 (2016).
Wahl, M. et al. Chemotherapy for adult low-grade gliomas: clinical outcomes by molecular subtype in a phase II study of adjuvant temozolomide. Neuro Oncol. 19, 242–251 (2016).
Kunwar, S. et al. Phase III randomized trial of CED of IL13-PE38QQR versus Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 12, 871–881 (2010).
Brem, H. et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J. Neurosurg. 74, 441–446 (1991).
Prados, M. D. et al. Interstitial brachytherapy for newly diagnosed patients with malignant gliomas: the UCSF experience. Int. J. Radiat. Oncol. Biol. Phys. 24, 593–597 (1992).
Cloughesy, T. F. et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl Med. 8, 341ra75 (2016).
Tetard, M. C. et al. Interstitial 5-ALA photodynamic therapy and glioblastoma: preclinical model development and preliminary results. Photodiagnosis Photodyn. Ther. 13, 218–224 (2016).
LoRusso, P. M. Phase 0 clinical trials: an answer to drug development stagnation? J. Clin. Oncol. 27, 2586–2588 (2009).
Wakana, S., Jiang, H., Nagae-Poetscher, L. M., van Zijl, P. C. & Mori, S. Fiber tract-based atlas of human white matter anatomy. Radiology 230, 77–87 (2004).
Mickevicius, N. J. et al. Location of brain tumor intersecting white matter tracts predicts patient prognosis. J. Neurooncol. 125, 393–400 (2015).
Nimsky, C., Bauer, M. & Carl, B. Merits and limits of tractography techniques for the uninitiated. Adv. Tech. Stand. Neurosurg. 43, 37–60 (2016).
McDonald, C. R. et al. Recovery of white matter tracts in regions of peritumoral FLAIR hyperintensity with use of restriction spectrum imaging. AJNR Am. J. Neuroradiol. 34, 1157–1163 (2013).
Picht, T. Current and potential utility of transcranial magnetic stimulation in the diagnostics before brain tumor surgery. CNS Oncol. 3, 299–310 (2014).
Picht, T., Frey, D., Thieme, S., Kliesch, S. & Vajkoczy, P. Presurgical navigated TMS motor cortex mapping improves outcome in glioblastoma surgery: a controlled observational study. J. Neurooncol. 126, 535–543 (2016).
Frey, D. et al. Navigated transcranial magnetic stimulation improves the treatment outcome in patients with brain tumors in motor eloquent locations. Neuro Oncol. 16, 1365–1372 (2014).
Picht, T., Schulz, J. & Vajkoczy, P. The preoperative use of navigated transcranial magnetic stimulation facilitates early resection of suspected low-grade gliomas in the motor cortex. Acta Neurochir. 155, 1813–1821 (2013).
Krieg, S. M. et al. Preoperative motor mapping by navigated transcranial magnetic brain stimulation improves outcome for motor eloquent lesions. Neuro Oncol. 16, 1274–1282 (2014).
Coburger, J. et al. Comparison of navigated transcranial magnetic stimulation and functional magnetic resonance imaging for preoperative mapping in rolandic tumor surgery. Neurosurg. Rev. 36, 65–75 (2013).
Ranck, J. B. Jr. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 98, 417–440 (1975).
Kunieda, T., Yamao, Y., Kikuchi, T. & Matsumoto, R. New approach for exploring cerebral functional connectivity: review of cortico-cortical evoked potential. Neurol. Med. Chir. 55, 374–382 (2015).
Haglund, M. M., Ojemann, G. A. & Blasdel, G. G. Optical imaging of bipolar cortical stimulation. J. Neurosurg. 78, 785–793 (1993).
Herholz, K. et al. Individual functional anatomy of verb generation. Neuroimage 3, 185–194 (1996).
Ojemann, G., Ojemann, J., Lettich, E. & Berger, M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J. Neurosurg. 71, 316–326 (1989).
Ojemann, G. A. & Whitaker, H. A. Language localization and variability. Brain Lang. 6, 239–260 (1978).
Ojemann, G. A. Individual variability in cortical localization of language. J. Neurosurg. 50, 164–169 (1979).
Glasser, M. F. et al. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 (2016).
Ojemann, J. G., Miller, J. W. & Silbergeld, D. L. Preserved function in brain invaded by tumor. Neurosurgery 39, 253–258 (1996).
Seitz, R. J. et al. Large-scale plasticity of the human motor cortex. Neuroreport 6, 742–744 (1995).
Wunderlich, G. et al. Precentral glioma location determines the displacement of cortical hand representation. Neurosurgery 42, 18–26 (1998).
Robles, S. G., Gatignol, P., Lehericy, S. & Duffau, H. Long-term brain plasticity allowing a multistage surgical approach to World Health Organization grade II gliomas in eloquent areas. J. Neurosurg. 109, 615–624 (2008).
Duffau, H. Brain plasticity: from pathophysiological mechanisms to therapeutic applications. J. Clin. Neurosci. 13, 885–897 (2006).
Duffau, H., Taillandier, L., Gatignol, P. & Capelle, L. The insular lobe and brain plasticity: Lessons from tumor surgery. Clin. Neurol. Neurosurg. 108, 543–548 (2006).
Tate, M. C., Herbet, G., Moritz-Gasser, S., Tate, J. E. & Duffau, H. Probabilistic map of critical functional regions of the human cerebral cortex: Broca's area revisited. Brain 137, 2773–2782 (2014).
Chang, E. F. et al. Stereotactic probability and variability of speech arrest and anomia sites during stimulation mapping of the language dominant hemisphere. J. Neurosurg. 126, 114–121 (2016).
Quinones-Hinojosa, A., Ojemann, S. G., Sanai, N., Dillon, W. P. & Berger, M. S. Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. J. Neurosurg. 99, 311–318 (2003).
Seghier, M. L. et al. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum. Brain Mapp. 23, 140–155 (2004).
Tzourio-Mazoyer, N., Josse, G., Crivello, F. & Mazoyer, B. Interindividual variability in the hemispheric organization for speech. Neuroimage 21, 422–435 (2004).
Turkeltaub, P. E., Eden, G. F., Jones, K. M. & Zeffiro, T. A. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16, 765–780 (2002).
Tzourio, N., Crivello, F., Mellet, E., Nkanga-Ngila, B. & Mazoyer, B. Functional anatomy of dominance for speech comprehension in left handers versus right handers. Neuroimage 8, 1–16 (1998).
Dehaene, S. et al. Anatomical variability in the cortical representation of first and second language. Neuroreport 8, 3809–3815 (1997).
Steinmetz, H. & Seitz, R. J. Functional anatomy of language processing: neuroimaging and the problem of individual variability. Neuropsychologia 29, 1149–1161 (1991).
Josse, G., Herve, P. Y., Crivello, F., Mazoyer, B. & Tzourio-Mazoyer, N. Hemispheric specialization for language: brain volume matters. Brain Res. 1068, 184–193 (2006).
FitzGerald, D. B. et al. Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. AJNR Am. J. Neuroradiol. 18, 1529–1539 (1997).
Skirboll, S. S., Ojemann, G. A., Berger, M. S., Lettich, E. & Winn, H. R. Functional cortex and subcortical white matter located within gliomas. Neurosurgery 38, 678–684 (1996).
Duffau, H. Stimulation mapping of white matter tracts to study brain functional connectivity. Nat. Rev. Neurol. 11, 255–265 (2015).
Moritz-Gasser, S., Herbet, G., Maldonado, I. L. & Duffau, H. Lexical access speed is significantly correlated with the return to professional activities after awake surgery for low-grade gliomas. J. Neurooncol. 107, 633–641 (2012).
Wengenroth, M. et al. Diagnostic benefits of presurgical fMRI in patients with brain tumours in the primary sensorimotor cortex. Eur. Radiol. 21, 1517–1525 (2011).
Cochereau, J. et al. Comparison between resting state fMRI networks and responsive cortical stimulations in glioma patients. Hum. Brain Mapp. 37, 3721–3732 (2016).
Romstock, J., Fahlbusch, R., Ganslandt, O., Nimsky, C. & Strauss, C. Localisation of the sensorimotor cortex during surgery for brain tumours: feasibility and waveform patterns of somatosensory evoked potentials. J. Neurol. Neurosurg. Psychiatry. 72, 221–229 (2002).
Sanai, N., Mirzadeh, Z. & Berger, M. S. Functional outcome after language mapping for glioma resection. N. Engl. J. Med. 358, 18–27 (2008).
Taylor, M. D. & Bernstein, M. Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: a prospective trial of 200 cases. J. Neurosurg. 90, 35–41 (1999).
Rolston, J. D. et al. Frontal operculum gliomas: language outcome following resection. J. Neurosurg. 122, 725–734 (2015).
Duffau, H. et al. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J. Neurol. Neurosurg. Psychiatry. 74, 901–907 (2003).
Thiel, A. et al. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann. Neurol. 50, 620–629 (2001).
Duffau, H., Bauchet, L., Lehericy, S. & Capelle, L. Functional compensation of the left dominant insula for language. Neuroreport 12, 2159–2163 (2001).
Fandino, J., Kollias, S. S., Wieser, H. G., Valavanis, A. & Yonekawa, Y. Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns in patients with brain tumors involving the primary motor cortex. J. Neurosurg. 91, 238–250 (1999).
Southwell, D. G., Hervey-Jumper, S. L., Perry, D. W. & Berger, M. S. Intraoperative mapping during repeat awake craniotomy reveals the functional plasticity of adult cortex. J. Neurosurg. 124, 1460–1469 (2016).
De Witt Hamer, P. C., Gil Robles, S., Zwinderman, A. H., Duffau, H. & Berger, M. S. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J. Clin. Oncol. 30, 2559–2565 (2012).
Klein, M., Duffau, H. & De Witt Hamer, P. C. Cognition and resective surgery for diffuse infiltrative glioma: an overview. J. Neurooncol. 108, 309–318 (2012).
Klein, M. Health-related quality of life aspects in patients with low-grade glioma. Adv. Tech. Stand. Neurosurg. 35, 213–235 (2010).
Cochereau, J., Herbet, G. & Duffau, H. Patients with incidental WHO grade II glioma frequently suffer from neuropsychological disturbances. Acta Neurochir. 158, 305–312 (2016).
Salcman, M. & Samaras, G. M. Hyperthermia for brain tumors: biophysical rationale. Neurosurgery 9, 327–335 (1981).
Voigt, J. D. & Barnett, G. The value of using a brain laser interstitial thermal therapy (LITT) system in patients presenting with high grade gliomas where maximal safe resection may not be feasible. Cost. Eff. Resour. Alloc. 14, 6 (2016).
Patel, P., Patel, N. V. & Danish, S. F. Intracranial MR-guided laser-induced thermal therapy: single-center experience with the Visualase thermal therapy system. J. Neurosurg. 125, 853–860 (2016).
Gasser, T. et al. Intraoperative functional MRI: implementation and preliminary experience. NeuroImage 26, 685–693 (2005).
Abernethy, L. J., Avula, S., Hughes, G. M., Wright, E. J. & Mallucci, C. L. Intra-operative 3-T MRI for paediatric brain tumours: challenges and perspectives. Pediatr. Radiol. 42, 147–157 (2012).
Caverzasi, E. et al. Identifying preoperative language tracts and predicting postoperative functional recovery using HARDI q-ball fiber tractography in patients with gliomas. J. Neurosurg. 125, 33–45 (2016).
Paiva, W. S., Fonoff, E. T., Marcolin, M. A., Cabrera, H. N. & Teixeira, M. J. Cortical mapping with navigated transcranial magnetic stimulation in low-grade glioma surgery. Neuropsychiatr. Dis. Treat. 8, 197–201 (2012).
Hayashi, Y., Nakada, M., Kinoshita, M. & Hamada, J-i. Functional reorganization in the patient with progressing glioma of the pure primary motor cortex: a case report with special reference to the topographic central sulcus defined by somatosensory-evoked potential. World Neurosurg. 82, 536.e1–536.e4 (2014).
Mohammadi, A. M. et al. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med. 3, 971–979 (2014).
Thomas, J. G., Rao, G., Kew, Y. & Prabhu, S. S. Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurg. Focus 41, E12 (2016).
Pessina, F. et al. Value of surgical resection in patients with newly diagnosed grade III glioma treated in a multimodal approach: surgery, chemotherapy and radiotherapy. Ann. Surg. Oncol. 23, 3040–3046 (2016).
Snyder, L. A. et al. The impact of extent of resection on malignant transformation of pure oligodendrogliomas. J. Neurosurg. 120, 309–314 (2014).
Author information
Authors and Affiliations
Contributions
N.S. researched the data for the article. Both authors contributed to discussions of content and wrote, reviewed, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sanai, N., Berger, M. Surgical oncology for gliomas: the state of the art. Nat Rev Clin Oncol 15, 112–125 (2018). https://doi.org/10.1038/nrclinonc.2017.171
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2017.171
This article is cited by
-
Development of preoperative and postoperative models to predict recurrence in postoperative glioma patients: a longitudinal cohort study
BMC Cancer (2024)
-
Application of PET imaging delta radiomics for predicting progression-free survival in rare high-grade glioma
Scientific Reports (2024)
-
Soft and stretchable organic bioelectronics for continuous intraoperative neurophysiological monitoring during microsurgery
Nature Biomedical Engineering (2023)
-
A validated prognostic nomogram for patients with H3 K27M-mutant diffuse midline glioma
Scientific Reports (2023)
-
Oligodendrogliomas tend to infiltrate the frontal aslant tract, whereas astrocytomas tend to displace it
Neuroradiology (2023)