Abstract
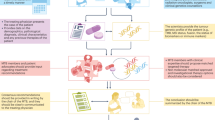
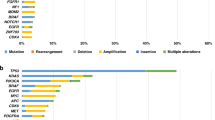
Patients with cancers of differing histologies that express certain biomarkers are likely to benefit from treatment with targeted therapies. However, targets can be present in malignancies other than those indicated by a drug's label, and as a result, affected patients will have no access to those potentially useful drugs. To tackle this issue, the French National Cancer Institute developed the AcSé Programme in 2013. This programme is designed to make treatment decisions or recommendations on the basis of the presence of relevant biomarkers for malignancies with no targeted therapies available and also aims to improve safety, and evaluate the efficacy of targeted drugs used outside of their approved indications. Patients across France have access to molecular testing in 28 molecular genetics centres and to targeted therapies within phase II trials provided no other trials exist in which they could reasonably be included. Trials include patients below the age of 18 if safe dosing data are available. As of January 2016, 183 French clinical sites and over 7,000 patients are participating in AcSé led trials. Proof of concept is being demonstrated through trials designed to investigate the effectiveness of crizotinib and vemurafenib in a wide variety of cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Institut National du Cancer. Situation sur la chimothérapie des cancers: rapport 2014. http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Rechercher-des-publications?theme=50709&type_cancer=&public=&year=&langue=&collection=
Bertolini, F., Sukhatme, V. P. & Bouche, G. Drug repurposing in oncology — patient and health systems opportunities. Nat. Rev. Clin. Oncol. 12, 732–742 (2015).
De Souza, J. A. et al. Unsupported off-label chemotherapy in metastatic colon cancer. BMC Health Serv. Res. 29, 481 (2012).
Casali, P. G., Bruzzi, P., Bogaerts, J. & Blay, J.-Y. Rare cancers in Europe (RCE) methodological recommendations for clinical studies in rare cancers: a European consensus position paper. Ann. Oncol. 26, 300–306 (2015).
Nowak, F., Soria, J. C. & Calvo, F. Tumour molecular profiling for deciding therapy — the French initiative. Nat. Rev. Clin. Oncol. 9, 479–486 (2012).
SIOPe. The SIOP-Europe strategic plan: a European cancer plan for children and adolescents. [online], (2015).
US National Library of Science. ClinicalTrials.gov[online], (2016).
US National Library of Science. ClinicalTrials.gov[online], (2015).
US National Library of Science. ClinicalTrials.gov[online], (2015).
US National Library of Science. ClinicalTrials.gov[online], (2015).
US National Library of Science. ClinicalTrials.gov[online], (2016).
Cui, J. J. et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J. Med. Chem. 54, 6342–6363 (2011).
Kwak, E. L. et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 363, 1693–1703 (2010).
Mano, H. ALKoma: a cancer subtype with a shared target. Cancer Discov. 2, 495–502 (2012).
Carpentier, E. L. & Mossé, Y. P. Targeting ALK in neuroblastoma-preclinical and clinical advancements. Nat. Rev. Clin. Oncol. 9, 391–399 (2012).
Gherardi, E., Birchmeier, W., Birchmeier, C. & Vande Woude, G. Targeting MET in cancer: rationale and progress. Nat. Rev. Cancer 12, 89–103 (2012).
Ou, S. H., Tan, J., Yen, Y. & Soo, R. A. ROS1 as a 'druggable' receptor tyrosine kinase: lessons learned from inhibiting the ALK pathway. Expert Rev. Anticancer Ther. 12, 447–456 (2014).
Gaudino, G. et al. RON is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 13, 3524–3532 (1994).
Chiarle, R., Voena, C., Ambrogio, C., Piva, R. & Inghirami, G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat. Rev. Cancer 8, 11–23 (2008).
Shaw, A. T., Hsu, P. P., Awad, M. M. & Engelman, J. A. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat. Rev. Cancer 13, 772–787 (2013).
van Gaal, J. C. et al. Anaplastic lymphoma kinase aberrations in rhabdomyosarcoma: clinical and prognostic implications. J. Clin. Oncol. 30, 308–315 (2012).
Ma, P. C. et al. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes Cancer 47, 1025–1037 (2008).
Tuma, R. S. ALK gene amplified in most inflammatory breast cancers. J. Natl Cancer Inst. 104, 87–88 (2012).
Schmidt, L. et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 16, 68–73 (1997).
Pierscianek, D. et al. MET gain in diffuse astrocytomas is associated with poorer outcome. Brain Pathol. 23, 13–18 (2013).
Charest, A. I. et al. Fusion of FIG to the receptor tyrosine kinase ROS in a glioblastoma with an interstitial del(6)(q21q21). Genes Chromosomes Cancer 37, 58–71 (2003).
Karayan-Tapon, L. et al. Lack of GOPC–ROS1 (FIG–ROS1) rearrangement in adult human gliomas. Eur. J. Cancer 50, 2364–2366 (2014).
Butrynski, J. E. et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N. Engl. J. Med. 363, 1727–1733 (2010).
Lovly, C. M. et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 4, 889–895 (2014).
Mossé, Y. P. et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase I consortium study. Lancet Oncol. 14, 472–480 (2013).
Vassal, G., Geoerger, B. & Morland, B. Is the European pediatric medicine regulation working for children and adolescents with cancer? Clin. Cancer Res. 19, 1315–1325 (2013).
US National Library of Science. ClinicalTrials.gov[online], (2016).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002).
Hall, R. D. & Kudchardkar, R. R. BRAF mutations: signalling, epidemiology, and clinical experience in multiple malignancies. Cancer Control 21, 221–229 (2014).
Bautista, F. et al. Vemurafenib in pediatric patients with BRAFV600E mutated high-grade gliomas. Pediatr. Blood Cancer 61, 1101–1103 (2014).
US National Library of Science. ClinicalTrials.gov[online], (2016).
Hyman, D. M. et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 373, 726–736 (2015).
Sinha, R. et al. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanomas: practical advice on diagnosis, prevention and management of the main treatment-related skin toxicities. Br. J. Dermatol. 167, 987–994 (2012).
Simon, R. Optimal two-stage designs for phase II clinical trials. Control Clin. Trials 10, 1–10 (1989).
Gehan, E. A. The determination of the number of patients required in a preliminary and a follow-up trial of a new chemotherapeutic agent. J. Chron. Dis. 13, 346–353 (1961).
Ensign, L. G., Gehan, E. A., Kamen, D. S. & Thal, P. F. An optimal three-stage design for phase II clinical trials. Stat. Med. 13, 1727–1736 (1994).
Zohar, S., Teramukai, S. & Zhou, Y. Bayesian design and conduct of phase II single-arm clinical trials with binary outcomes: a tutorial. Contemp. Clin. Trials 29, 608–616 (2008).
Committee for medicinal products for human use (CHMP). Guideline on clinical trials in small populations. European Medical Agency [online], (2006).
Mayo, M. S. & Gajewski, B. J. Bayesian sample size calculations in phase II clinical trials using informative conjugate priors. Control Clin. Trials 25, 157–167 (2004).
Neuenschwander, B., Capkun-Niggli, G., Branson, M. & Spiegelhalter, D. J. Summarizing historical information on controls in clinical trials. Clin. Trials 7, 5–18 (2010).
El-Maraghi, R. H. & Eisenhauer, E. A. Review of phase II trial designs used in studies of molecular targeted agents: outcomes and predictors of success in phase III. J. Clin. Oncol. 26, 1346–1354 (2008).
US National Library of Science. ClinicalTrials.gov[online], (2015).
Agence nationalle de Sécurité du Médicament et des produtis de santé. Clinical trials registry. [online]
Schilsky, R. L. ASCO's Targeted Agent and Profiling Utilization Registry (TAPUR) Study. ASCO [online], (2016).
André, F. et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol. 15, 267–274 (2014).
Ferté, C. et al. Molecular screening for cancer treatment optimization (MOSCATO 01): a prospective molecular triage trial [abstract]. Cancer Res. 74 (Suppl.), CT240 (2014).
Tsimberidou, A. M. et al. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: validation and landmark analyses. Clin. Cancer Res. 20, 4827–4836 (2014).
Conley, B. A. & Doroshow, J. H. Molecular analysis for therapy choice: NCI MATCH. Semin. Oncol. 41, 297–299 (2014).
Kang, B. P. et al. The Signature Program: bringing the protocol to the patient. Clin. Pharmacol. Ther. 98, 124–126 (2015).
Conti, R. M. et al. Prevalence of off-label use and spending in 2010 among patent-protected chemotherapies in a population-based cohort of medical oncologsts. J. Clin. Oncol. 31, 1134–1139 (2013).
Joerger, M. et al. Off-label use of anticancer drugs in eastern Switzerland: a population-based prospective cohort study. Eur. J. Clin. Pharmacol. 70, 719–725 (2014).
Mehr, S. R. The complexity of covering off-label use for a multitude of oncology regimens. Am. J. Manag. Care 18, SP242–SP247 (2012).
Irwin, B., Hirsch, B. R., Samsa, G. P. & Abernethy, A. P. Conflict of interest disclosure in off-label oncology clinical trials. J. Oncol. Pract. 8, 298–302 (2012).
Eberst, L. et al. The off-label use of targeted therapies in sarcomas: the OUTC'S program. BMC Cancer 24, 870 (2014).
Acknowledgements
We thank Maren White (freelancer editor and medical writer) for her editorial support in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
N. H-L., M. J., F. N., M-C.L-D., D. P. and G. V. researched data for this article. M-C.L-D., D. P. and G. V. wrote the article, A. B., J.-Y. B., M-C.L-D., D. P. and G. V. made a substantial contribution to the discussion of content, and all authors review and edited the manuscript before submission and during revisions.
Corresponding author
Ethics declarations
Competing interests
The AcSé programme is funded by INCA and ARC Foundation for Cancer Research. The clinical trials are sponsored by Unicancer. G.V. is chief-investigator of AcSé-crizotinib and J.-Y. B. is chief-investigator of AcSé-vémurafénib.
Rights and permissions
About this article
Cite this article
Buzyn, A., Blay, JY., Hoog-Labouret, N. et al. Equal access to innovative therapies and precision cancer care. Nat Rev Clin Oncol 13, 385–393 (2016). https://doi.org/10.1038/nrclinonc.2016.31
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.31
This article is cited by
-
The Activity of Crizotinib in Chemo-Refractory MET-Amplified Esophageal and Gastric Adenocarcinomas: Results from the AcSé-Crizotinib Program
Targeted Oncology (2021)
-
Genomic profiling in oncology clinical practice
Clinical and Translational Oncology (2020)
-
Long-term efficacy of crizotinib in a metastatic papillary renal carcinoma with MET amplification: a case report and literature review
BMC Cancer (2018)