Key Points
-
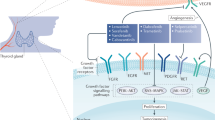
Advances in understanding the genomic and functional alterations contributing to the pathogenesis of thyroid cancers have opened new therapeutic opportunities and are beginning to improve patient outcomes
-
RET mutations are common in medullary thyroid cancers (MTCs), and the multikinase inhibitors vandetanib and cabozantinib, which inhibit RET as well as other kinases, are now approved treatments for this disease
-
Multiple small-molecule multikinase inhibitors that block VEGFR signalling have shown promise in the treatment of radioactive iodine (RAI)-refractory differentiated thyroid cancer (DTC): sorafenib and lenvatinib are approved for use in the metastatic setting
-
Use of multikinase inhibitors can result in substantial toxicity, a 1–3% risk of death, and does not conclusively improve overall survival; these agent should thus be used with considerable restraint
-
MAPK-pathway inhibition might improve RAI uptake and, therefore, patient responses to RAI therapy with iodine-131; this combinatorial therapeutic approach is a promising novel strategy in patients with metastatic DTC
-
Early application of intensive multimodality therapy (combining surgery, intensity-modulated radiotherapy and taxane-based chemotherapy) might prolong overall survival of patients with anaplastic thyroid cancer, especially those with early stage disease
Abstract
Increased understanding of disease-specific molecular targets of therapy has led to the regulatory approval of two drugs (vandetanib and cabozantinib) for the treatment of medullary thyroid cancer (MTC), and two agents (sorafenib and lenvatinib) for the treatment of radioactive- iodine refractory differentiated thyroid cancer (DTC) in both the USA and in the EU. The effects of these and other therapies on overall survival and quality of life among patients with thyroid cancer, however, remain to be more-clearly defined. When applied early in the disease course, intensive multimodality therapy seems to improve the survival outcomes of patients with anaplastic thyroid cancer (ATC), but salvage therapies for ATC are of uncertain benefit. Additional innovative, rationally designed therapeutic strategies are under active development both for patients with DTC and for patients with ATC, with multiple phase II and phase III randomized clinical trials currently ongoing. Continued effort is being made to identify further signalling pathways with potential therapeutic relevance in thyroid cancers, as well as to elaborate on the complex interactions between signalling pathways, with the intention of translating these discoveries into effective and personalized therapies. Herein, we summarize the progress made in molecular medicine for advanced-stage thyroid cancers of different histotypes, analyse how these developments have altered — and might further refine — patient care, and identify open questions for future research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
American Cancer Society. Cancer facts & figures 2015. cancer.org[online], (2015).
Chen, A. Y., Jemal, A. & Ward, E. M. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 115, 3801–3807 (2009).
Enewold, L. et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol. Biomarkers Prev. 18, 784–791 (2009).
Hayat, M. J., Howlader, N., Reichman, M. E. & Edwards, B. K. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 12, 20–37 (2007).
Jemal, A. et al. Cancer statistics, 2009. CA Cancer J. Clin. 59, 225–249 (2009).
Davies, L. & Welch, H. G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295, 2164–2167 (2006).
Al-Eid, H. S. & Arteh, S. O. Cancer incidence report Saudi Arabia 2003. Saudi Health Council [online], (2003).
Hussain, F. et al. Incicidence of thyroid cancer in the Kingdom of Saudi Arabia, 2000–2010. Hematol. Oncol. Stem Cell Ther. 6, 58–64 (2013).
Ahn, H. S., Kim, H. J. & Welch, H. G. Korea's thyroid-cancer 'epidemic' — screening and overdiagnosis. N. Engl. J. Med. 371, 1765–1767 (2014).
Brito, J. P., Al Nofal, A., Montori, V. M., Hay, I. D. & Morris, J. C. The impact of subclinical disease and mechanism of detection on the rise in thyroid cancer incidence: a population-based study in Olmsted County, Minnesota during 1935 through 2012. Thyroid 25, 999–1007 (2015).
Aschebrook-Kilfoy, B. et al. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol. Biomarkers Prev. 22, 1252–1259 (2013).
Ramsey, S. et al. Washington state cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff. (Millwood) 32, 1143–1152 (2013).
Smallridge, R. C. & Copland, J. A. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin. Oncol. (R. Coll. Radiol.) 22, 486–497 (2010).
Brat, D. J. et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159, 676–690 (2014).
Surov, A. et al. Primary thyroid sarcoma: a systematic review. Anticancer Res. 35, 5185–5191 (2015).
Chai, Y. J. et al. Clinicopathological characteristics and treatment outcomes of 38 cases of primary thyroid lymphoma: a multicenter study. Ann. Surg. Treat. Res. 89, 295–299 (2015).
Harris, P. J. & Bible, K. C. Emerging therapeutics for advanced thyroid malignancies: rationale and targeted approaches. Expert Opin. Investig. Drugs 20, 1357–1375 (2011).
Hancock, S. L., Cox, R. S. & McDougall, I. R. Thyroid diseases after treatment of Hodgkin's disease. N. Engl. J. Med. 325, 599–605 (1991).
Jacob, P. et al. Thyroid cancer risk in areas of Ukraine and Belarus affected by the Chernobyl accident. Radiat. Res. 165, 1–8 (2006).
Ahn, D. et al. Clinical relationship between Hashimoto's thyroiditis and papillary thyroid cancer. Acta Oncol. 50, 1228–1234 (2011).
Wang, X. et al. Endocrine tumours: familial nonmedullary thyroid carcinoma is a more aggressive disease: a systematic review and meta-analysis. Eur. J. Endocrinol. 172, R253–R262 (2015).
Navas-Carrillo, D., Ríos, A., Rodríguez, J. M., Parrilla, P. & Orenes-Piñero, E. Familial nonmedullary thyroid cancer: screening, clinical, molecular and genetic findings. Biochim. Biophys. Acta 1846, 468–476 (2014).
Gara, S. K. et al. Germline HABP2 mutation causing familial nonmedullary thyroid cancer. N. Engl. J. Med. 373, 448–455 (2015).
Katoh, H., Yamashita, K., Enomoto, T. & Watanabe, M. Classification and general considerations of thyroid cancer. Ann. Clin. Pathol. 3, 1045 (2015).
Tuttle, R. M. et al. Thyroid carcinoma, version 2.2014. J. Natl Compr. Canc Netw. 12, 1671–1680 (2014).
Ganly, I. et al. Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid malignancy. J. Clin. Endocrinol. Metab. 98, E962–E972 (2013).
Chindris, A. M. et al. Clinical and molecular features of Hürthle cell carcinoma of the thyroid. J. Clin. Endocrinol. Metab. 100, 55–62 (2015).
Wells, S. A. Jr et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma: the american thyroid association guidelines task force on medullary thyroid carcinoma. Thyroid 25, 567–610 (2015).
Nikiforov, Y. E. & Nikiforova, M. N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 7, 569–580 (2011).
Aschebrook-Kilfoy, B., Ward, M. H., Sabra, M. M. & Devesa, S. S. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 21, 125–134 (2011).
Spitzweg, C., Bible, K. C., Hofbauer, L. C. & Morris, J. C. Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol. 2, 830–842 (2014).
Haugen, B. R. et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 26, 1–133 (2016).
Schlumberger, M. et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2, 356–358 (2014).
Durante, C. et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 91, 2892–2899 (2006).
Sabra, M. M. et al. Clinical outcomes and molecular profile of differentiated thyroid cancers with radioiodine-avid distant metastases. J. Clin. Endocrinol. Metab. 98, E829–E836 (2013).
Albero, A., Lopéz, J. E., Torres, A., de la Cruz, L. & Martín, T. Effectiveness of chemotherapy in advanced differentiated thyroid cancer: a systematic review. Endocr. Relat. Cancer 23, R71–R84 (2016).
Spencer, C. A., LoPresti, J. S., Fatemi, S. & Nicoloff, J. T. Detection of residual and recurrent differentiated thyroid carcinoma by serum thyroglobulin measurement. Thyroid 9, 435–441 (1999).
Hoofnagle, A. N. & Roth, M. Y. Clinical review: improving the measurement of serum thyroglobulin with mass spectrometry. J. Clin. Endocrinol. Metab. 98, 1343–1352 (2013).
Camargo, R. Y. & Tomimori, E. K. Usefulness of ultrasound in the diagnosis and management of well-differentiated thyroid carcinoma. Arq. Bras. Endocrinol. Metabol. 51, 783–792 (2007).
Ishiwata, T., Iino, Y., Takei, H., Oyama, T. & Morishita, Y. Tumor angiogenesis as an independent prognostic indicator in human papillary thyroid carcinoma. Oncol. Rep. 5, 1343–1348 (1998).
Soh, E. Y. et al. Thyroid-stimulating hormone promotes the secretion of vascular endothelial growth factor in thyroid cancer cell lines. Surgery 120, 944–947 (1996).
Lennard, C. M. et al. Intensity of vascular endothelial growth factor expression is associated with increased risk of recurrence and decreased disease-free survival in papillary thyroid cancer. Surgery 129, 552–558 (2001).
Klein, M. et al. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 86, 656–658 (2001).
Yu, X. M. et al. Increased expression of vascular endothelial growth factor C in papillary thyroid carcinoma correlates with cervical lymph node metastases. Clin. Cancer Res. 11, 8063–8069 (2005).
Soh, E. Y. et al. Neutralizing vascular endothelial growth factor activity inhibits thyroid cancer growth in vivo. Surgery 128, 1059–1065 (2000).
Carlomagno, F. et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 62, 7284–7290 (2002).
Leboulleux, S. et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 13, 897–905 (2012).
Brose, M. S. et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384, 319–328 (2014).
Schlumberger, M. et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 372, 621–630 (2015).
Sherman, E. J. et al. A phase II study of VEGF trap in patients with radioactive iodine (RAI)-refractory thyroid carcinoma [abstract]. J. Clin. Oncol. 29, 5566 (2011).
Baxi, S. S. et al. Hemorrhagic pseudoaneurysm in a patient receiving aflibercept for metastatic thyroid cancer. Thyroid 22, 552–555 (2012).
US National Library of Science. ClinicalTrials.gov[online], (2015).
Schlumberger, M. et al. Randomized, double-blinded, placebo controled trial of sorafenib in locally advanced or metastatic patients with radioactive iodine (RAI)-refractory differentiated thyroid cancer (DTC) — exploratory analyses of patient-reported outcomes [abstract 100]. Presented at the 83rd Annual Meeting of the American Thyroid Association (2013).
Kimura, E. T. et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC–RAS–BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 63, 1454–1457 (2003).
Ricarte-Filho, J. C. et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 69, 4885–4893 (2009).
Chen, X., Makarewicz, J. M., Knauf, J. A., Johnson, L. K. & Fagin, J. A. Transformation by HrasG12V is consistently associated with mutant allele copy gains and is reversed by farnesyl transferase inhibition. Oncogene 33, 5442–5449 (2014).
Rao, S. et al. Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J. Clin. Oncol. 22, 3950–3957 (2004).
Heymach, J. V. et al. Phase II study of the farnesyl transferase inhibitor R115777 in patients with sensitive relapse small-cell lung cancer. Ann. Oncol. 15, 1187–1193 (2004).
Van Cutsem, E. et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J. Clin. Oncol. 22, 1430–1438 (2004).
US National Library of Science. ClinicalTrials.gov[online], (2015).
Montero-Conde, C. et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 3, 520–533 (2013).
US National Library of Science. ClinicalTrials.gov[online], (2015).
Kim, K. B. et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAFV600E mutation. Thyroid 23, 1277–1288 (2013).
Falchook, G. S. et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 379, 1893–1901 (2012).
Dadu, R. et al. Efficacy and tolerability of vemurafenib in patients with BRAFV600E-positive papillary thyroid cancer: M. D. Anderson Cancer Center off label experience. J. Clin. Endocrinol. Metab. 100, E77–E81 (2015).
Bible, K. C. et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 11, 962–972 (2010).
US National Library of Science. ClinicalTrials.gov[online], (2016).
Schlumberger, M. J. et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. Clin. Oncol. 27, 3794–3801 (2009).
Chakravarty, D. et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J. Clin. Invest. 121, 4700–4711 (2011).
Ho, A. L. et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N. Engl. J. Med. 368, 623–632 (2013).
Rothenberg, S. M. et al. V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin. Cancer Res. 21, 1028–1035 (2015).
Riesco-Eizaguirre, G. et al. The BRAFV600E oncogene induces transforming growth factor β secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res. 69, 8317–8325 (2009).
US National Library of Science. ClinicalTrials.gov[online], (2016).
US National Library of Science. ClinicalTrials.gov[online], (2015).
Reddi, H. V. et al. Antitumor activity of VB-111, a novel antiangiogenic virotherapeutic, in thyroid cancer xenograft mouse models. Genes Cancer 2, 993–995 (2011).
Jasim, S. et al. Multi-cohort phase II trial of VB-111 in advanced radioactive iodine-refractory differentiated thyroid cancer [abstract 638]. Presented at the 15th International Thyroid Congress and 85th Annual Meeting of the American Thyroid Association (2015).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Ribas, A. et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 16, 908–918 (2015).
Ryder, M., Callahan, M., Postow, M. A., Wolchok, J. & Fagin, J. A. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr. Relat. Cancer. 21, 371–381 (2014).
Cunha, L. L., Marcello, M. A. & Ward, L. S. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocr. Relat. Cancer 21, R85–R103 (2014).
Ryder, M., Ghossein, R. A., Ricarte-Filho, J. C., Knauf, J. A. & Fagin, J. A. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr. Relat. Cancer 15, 1069–1074 (2008).
Caillou, B. et al. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS ONE 6, e22567 (2011).
Ryder, M. et al. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLoS ONE 8, e54302 (2013).
Soda, M. et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature 448, 561–566 (2007).
Kelly, L. M. et al. Identification of the transforming STRN–ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc. Natl Acad. Sci. USA 111, 4233–4238 (2014).
Nikiforova, M. N. et al. RAS point mutations and PAX8-PPARγ rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J. Clin. Endocrinol. Metab. 88, 2318–2326 (2003).
McIver, B., Grebe, S. K. & Eberhardt, N. L. The PAX8/PPARγ fusion oncogene as a potential therapeutic target in follicular thyroid carcinoma. Curr. Drug Targets Immune Endocr. Metabol. Disord. 4, 221–234 (2004).
Dobson, M. E. et al. Pioglitazone induces a proadipogenic antitumor response in mice with PAX8–PPARγ fusion protein thyroid carcinoma. Endocrinology 152, 4455–4465 (2011).
Kebebew, E. et al. Results of rosiglitazone therapy in patients with thyroglobulin-positive and radioiodine-negative advanced differentiated thyroid cancer. Thyroid 19, 953–956 (2009).
Melo, M. et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 99, E754–E765 (2014).
Kaserer, K. et al. Sporadic versus familial medullary thyroid microcarcinoma: a histopathologic study of 50 consecutive patients. Am. J. Surg. Pathol. 25, 1245–1251 (2001).
Dvorakova, S. et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinomas. Mol. Cell Endocrinol. 284, 21–27 (2008).
Wells, S. A. Jr et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 30, 134–141 (2012).
Elisei, R. et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 31, 3639–3646 (2013).
Bible, K. C. et al. A multicenter phase 2 trial of pazopanib in metastatic and progressive medullary thyroid carcinoma: MC057H. J. Clin. Endocrinol. Metab. 99, 1687–1693 (2014).
Schlumberger, M. et al. Final overall survival analysis of EXAM, an international, double-blind, randomized, placebo-controlled phase III trial of cabozantinib (Cabo) in medullary thyroid carcinoma (MTC) patients with documented RECIST progression at baseline [abstract]. J. Clin. Oncol. 33, 6012 (2015).
Gild, M. L. et al. Targeting mTOR in RET mutant medullary and differentiated thyroid cancer cells. Endocr. Relat. Cancer 20, 659–667 (2013).
Chernock, R. D. & Hagemann, I. S. Molecular pathology of hereditary and sporadic medullary thyroid carcinomas. Am. J. Clin. Pathol. 143, 768–777 (2015).
Kumar, A. et al. Outcomes in response to aggressive multimodal therapy in anaplastic thyroid cancer: the Mayo Clinic Experience [abstract 72]. Presented at the 15th International Thyroid Congress and 85th Annual Meeting of the American Thyroid Association (2015).
Bible, K. C. et al. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 97, 3179–3184 (2012).
Ain, K. B., Egorin, M. J. & DeSimone, P. A. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Thyroid 10, 587–594 (2000).
Foote, R. L. et al. Enhanced survival in locoregionally confined anaplastic thyroid carcinoma: a single-institution experience using aggressive multimodal therapy. Thyroid 21, 25–30 (2011).
Sun, X. S. et al. Chemoradiation in anaplastic thyroid carcinomas. Crit. Rev. Oncol. Hematol. 86, 290–301 (2013).
Troch, M. et al. High efficacy of concomitant treatment of undifferentiated (anaplastic) thyroid cancer with radiation and docetaxel. J. Clin. Endocrinol. Metab. 95, E54–E57 (2010).
Ito, K. et al. Multimodality therapeutic outcomes in anaplastic thyroid carcinoma: improved survival in subgroups of patients with localized primary tumors. Head Neck 34, 230–237 (2012).
Swaak-Kragten, A. T., de Wilt, J. H., Schmitz, P. I., Bontenbal, M. & Levendag, P. C. Multimodality treatment for anaplastic thyroid carcinoma — treatment outcome in 75 patients. Radiother. Oncol. 92, 100–104 (2009).
Prasongsook, N. et al. Impact of aggressive combined-modality primary therapy in anaplastic thyroid carcinoma (ATC): an updated single-institution experience [abstract]. J. Clin. Oncol. 32, e17042 (2014).
Smallridge, R. C. et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 22, 1104–1139 (2012).
Dziba, J. M., Marcinek, R., Venkataraman, G., Robinson, J. A. & Ain, K. B. Combretastatin A4 phosphate has primary antineoplastic activity against human anaplastic thyroid carcinoma cell lines and xenograft tumors. Thyroid 12, 1063–1070 (2002).
Sosa, J. A. et al. Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid 24, 232–240 (2014).
Copland, J. A. et al. Novel high-affinity PPARγ agonist alone and in combination with paclitaxel inhibits human anaplastic thyroid carcinoma tumor growth via p21WAF1/CIP1. Oncogene 25, 2304–2317 (2006).
Marlow, L. A. et al. Reactivation of suppressed RhoB is a critical step for the inhibition of anaplastic thyroid cancer growth. Cancer Res. 69, 1536–1544 (2009).
Smallridge, R. C. et al. Efatutazone, an oral PPAR-γ agonist, in combination with paclitaxel in anaplastic thyroid cancer: results of a multicenter phase 1 trial. J. Clin. Endocrinol. Metab. 98, 2392–2400 (2013).
US National Library of Science. ClinicalTrials.gov[online], (2015).
Isham, C. R. et al. Pazopanib enhances paclitaxel-induced mitotic catastrophe in anaplastic thyroid cancer: role of aurora A and clinical translation. Sci. Transl. Med. 5, 1661a3 (2013).
US National Library of Science. ClinicalTrials.gov[online], (2016).
Rosove, M. H., Peddi, P. F. & Glaspy, J. A. BRAF V600E inhibition in anaplastic thyroid cancer. N. Engl. J. Med. 368, 684–685 (2013).
Godbert, Y. et al. Remarkable response to crizotinib in woman with anaplastic lymphoma kinase-rearranged anaplastic thyroid carcinoma. J. Clin. Oncol. 33, e84–e87 (2015).
Wagle, N. et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N. Engl. J. Med. 371, 1426–1433 (2014).
Takahashi, S. et al. Study of lenvatinib, a multitargeted tyrosine kinase inhibitor, in patients with all histologic subtypes of advanced thyroid cancer (differentiated, medullary, and anaplastic). Ann. Oncol. 25 (Suppl 4), iv340–iv356 (2014).
Roemeling, C. A. et al. Aberrant lipid metabolism in anaplastic thyroid carcinoma reveals stearoyl CoA desaturase 1 as a novel therapeutic target. J. Clin. Endocrinol. Metab. 100, E697–E709 (2015).
Kondo, T., Ezzat, S. & Asa, S. L. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat. Rev. Cancer 6, 292–306 (2006).
Smallridge, R. C., Marlow, L. A. & Copland, J. A. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr. Relat. Cancer 16, 17–44 (2009).
Author information
Authors and Affiliations
Contributions
K.C.B. and M.R. identified and evaluated literature and meeting presentations for inclusion in the article and wrote the manuscript. Both authors made substantial contributions to discussions of content and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bible, K., Ryder, M. Evolving molecularly targeted therapies for advanced-stage thyroid cancers. Nat Rev Clin Oncol 13, 403–416 (2016). https://doi.org/10.1038/nrclinonc.2016.19
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.19
This article is cited by
-
La PET/TC nell’era degli agenti citostatici: ruolo nella valutazione della risposta alla terapia con inibitori della tirosin-chinasi (TKI) nel carcinoma tiroideo
L'Endocrinologo (2024)
-
Draft genome sequence of Streptomyces sp. KD18, isolated from industrial soil
3 Biotech (2023)
-
Mutational status may supersede tumor size in predicting the presence of aggressive pathologic features in well differentiated thyroid cancer
Journal of Otolaryngology - Head & Neck Surgery (2022)
-
Adiponectin and leptin exert antagonizing effects on proliferation and motility of papillary thyroid cancer cell lines
Journal of Physiology and Biochemistry (2021)
-
Annexin A10 is a novel prognostic biomarker of papillary thyroid cancer
Irish Journal of Medical Science (1971 -) (2021)