Key Points
-
Oesophageal and gastric cancers are aggressive tumours that result in more than 1 million deaths annually worldwide
-
Oesophageal and gastric cancers harbour a high number of genetic and molecular alterations, some of which contribute to an aggressiveness phenotype resulting in early development of drug resistance
-
A new molecular classification of gastric cancer into four subtypes on the basis of genotypic, epigenetic and proteomic characteristics has been developed
-
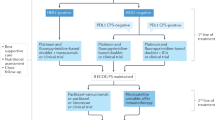
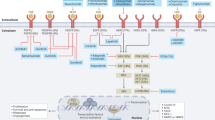
HER2-targeting with trastuzumab remains an important strategy in patients with HER2-positive, advanced-stage gastric cancer; however, novel anti-HER2 targeted drugs are being explored in the advanced-stage and perioperative treatment settings
-
Antiangiogenic treatment with ramucirumab has proved effective in a biologically unselected patient population with disease progression after first-line therapy
-
Treatments that target cancer stemness and immune-based therapies are two evolving concepts in the management of advanced-stage gastroesophageal cancer
Abstract
The characterization of oesophageal and gastric cancer into subtypes based on genotype has evolved in the past decade. Insights into the molecular landscapes of gastroesophageal cancer provide a roadmap to assist the development of new drugs and their use in combinations, for patient stratification, and for trials of targeted therapies. Trastuzumab is the only approved treatment for gastroesophageal cancers that overexpress HER2. Acquired resistance usually limits the duration of response to this treatment, although a number of new agents directed against HER2 have the potential to overcome or prolong the time until resistance occurs. Beyond that, anti-VEGFR2 therapy with ramucirumab was the first biological treatment strategy to produce a survival benefit in an unselected population of patients with chemotherapy-refractory gastroesophageal cancer. Large initiatives are starting to address the role of biomarker-driven targeted therapy in the metastatic and in the perioperative setting for patients with this disease. Immunotherapy also holds promise, and our understanding of subsets of gastroesophageal cancer based on patterns of immune response continues to evolve. Efforts are underway to identify more relevant genomic subsets through genomic screening, functional studies, and molecular characterization. Herein, we provide an overview of the key developments in the treatment of gastroesophageal cancer, and discuss potential strategies to further optimize therapy by targeting disease subtypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
de Martel, C. et al. Gastric cancer: epidemiology and risk factors. Gastroenterol. Clin. North Am. 42, 219–240 (2013).
Colquhoun, A. et al. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut 64, 1881–1888 (2015).
Kim, J. Y. et al. Lymph node metastasis in early gastric cancer: evaluation of a novel method for measuring submucosal invasion and development of a nodal predicting index. Hum. Pathol. 44, 2829–2836 (2013).
Gockel, I. et al. Risk of lymph node metastasis in submucosal esophageal cancer: a review of surgically resected patients. Expert Rev. Gastroenterol. Hepatol. 5, 371–384 (2011).
Wagner, A. D. et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 3, CD004064 (2010).
Lordick, F. et al. Optimal chemotherapy for advanced gastric cancer: is there a global consensus? Gastric Cancer 17, 213–225 (2014).
Van Cutsem, E. et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J. Clin. Oncol. 24, 4991–4997 (2006).
Van Cutsem, E. et al. Docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer: a randomized phase II study. Ann. Oncol. 26, 149–156 (2015).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Fuchs, C. S. et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383, 31–39 (2014).
Wilke, H. et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 15, 1224–1235 (2014).
Lordick, F. Gastrointestinal cancer: over the RAINBOW — renaissance in antiangiogenesis. Nat. Rev. Clin. Oncol. 12, 7–8 (2015).
Moehler, M. et al. International comparison of the German evidence-based S3-guidelines on the diagnosis and multimodal treatment of early and locally advanced gastric cancer, including adenocarcinoma of the lower esophagus. Gastric Cancer 18, 550–563 (2015).
National Comprehensive Cancer Network GuidelinesVersion 3.2015 Gastric Cancer. [online],
Lauren, P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol. Microbiol. Scand. 64, 31–49 (1965).
Marrelli, D. et al. Different patterns of recurrence in gastric cancer depending on Lauren's histologic type: longitudinal study. World J. Surg. 26, 1160–1165 (2002).
Carneiro, F. et al. Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and it's implications for patient screening. J. Pathol. 203, 681–687 (2004).
Yuo, W. C. et al. Precancerous gastric lesions in a population at high risk of stomach cancer. Cancer Res. 53, 1317–1321 (1993).
Correa, P. et al. Gastric precancerous process in a high risk population: cross-sectional studies. Cancer Res. 50, 4731–4736 (1990).
Anderson, W. F. et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 303, 1723–1728 (2010).
Blot, W. J. et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 265, 1287–1289 (1991).
Crew, K. D. & Neugut, A. I. Epidemiology of gastric cancer. World J. Gastroenterol. 12, 354–362 (2006).
Sakaguchi, T. et al. Characteristics and clinical outcome of proximal-third gastric cancer. J. Am. Coll. Surg. 187, 352–357 (1998).
Hofmann, M. et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52, 797–805 (2008).
Tafe, L. J. et al. Human epidermal growth factor receptor 2 testing in gastroesophageal cancer: correlation between immunohistochemistry and fluorescence in situ hybridization. Arch. Pathol. Lab. Med. 135, 1460–1465 (2011).
Steevens, J. et al. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur. J. Gastroenterol. Hepatol. 22, 669–678 (2010).
Marshall, B. J. & Warren, J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1, 1311–1314 (1984).
Correa, P. Helicobacter pylori and gastric carcinogenesis. Am. J. Surg. Pathol. 19, S37–S43 (1995).
Figueiredo, C. et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J. Natl Cancer Inst. 94, 1680–1687 (2002).
Yang, P. et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur. J. Cancer 45, 2867–2873 (2009).
Tsugane, S. & Sasazuki, S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer 10, 75–83 (2007).
Mendez, M. A. et al. Cereal fiber intake may reduce risk of gastric adenocarcinomas: the EPIC-EURGAST study. Int. J. Cancer 121, 1618–1623 (2007).
Lunet, N. et al. Fruit and vegetables consumption and gastric cancer: a systematic review and meta-analysis of cohort studies. Nutr. Cancer 53, 1–10 (2005).
Shibata, D. et al. Association of Epstein−Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am. J. Pathol. 139, 469–474 (1991).
Kusano, M. et al. Genetic, epigenetic, and clinicopathologic features of gastric carcinomas with the CpG island methylator phenotype and an association with Epstein–Barr virus. Cancer 106, 1467–1479 (2006).
Bass, A. J. et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209 (2014).
Cheng, D. T. et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 17, 251–264 (2015).
Memorial Sloan Kettering Cancer Center. cBioPortal for Cancer Genomics http://www.cbioportal.org/study.do?cancer_study_id=egc_tmucih_2015#summary
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013).
Riches, J. C. et al. Genomic profiling of esophagogastric (EG) tumors in clinical practice [abstract]. J. Clin. Oncol. 33 (Suppl. 3), 57 (2015).
Cristescu, R. et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 21, 449–456 (2015).
Janjigian, Y. Y. et al. Patient-derived xenografts as models for the identification of predictive biomarkers in esophagogastric cancer [abstract]. J. Clin. Oncol. 32 (Suppl. 5), 4059 (2014).
Fitzgerald, R. C. et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J. Med. Genet. 47, 436–444 (2010).
Guilford, P. et al. E-cadherin germline mutations in familial gastric cancer. Nature 392, 402–405 (1998).
Huntsman, D. G. et al. Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. N. Engl. J. Med. 344, 1904–1909 (2001).
Pharoah, P. D. et al. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 121, 1348–1353 (2001).
Benusiglio, P. R. et al. CDH1 germline mutations and the hereditary diffuse gastric and lobular breast cancer syndrome: a multicentre study. J. Med. Genet. 50, 486–489 (2013).
van der Post, R. S. et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J. Med. Genet. 52, 361–374 (2015).
Moran, A. et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Familial Cancer 11, 235–242 (2012).
Watanabe, H. et al. Gastric lesions in familial adenomatosis coli: their incidence and histologic analysis. Hum. Pathol. 9, 269–283 (1978).
Lynch, H. T. et al. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology 104, 1535–1549 (1993).
Jakubowska, A. et al. BRCA2 gene mutations in families with aggregations of breast and stomach cancers. Br. J. Cancer 87, 888–891 (2002).
Oliveira, C. et al. Genetic screening for hereditary diffuse gastric cancer. Expert Rev. Mol. Diagn. 3, 201–215 (2003).
Worthley, D. L. et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): a new autosomal dominant syndrome. Gut 61, 774–779 (2012).
Oliveira, C. et al. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 16, e60–e70 (2015).
Hansford, S. et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 1, 23–32 (2015).
Hoskins, L. C. et al. Distribution of ABO blood groups in patients with pernicious anemia, gastric carcinoma and gastric carcinoma associated with pernicious anemia. N. Engl. J. Med. 273, 633–637 (1965).
Edgren, G. et al. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: a cohort study. Am. J. Epidemiol. 172, 1280–1285 (2010).
Won, E. et al. HER2 directed therapy for gastric/esophageal cancers. Curr. Treat. Opt. Oncol. 15, 395–404 (2014).
Zhang, X. L. et al. Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J. Surg. 33, 2112–2118 (2009).
Begnami, M. D. et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J. Clin. Oncol. 29, 3030–3036 (2011).
Warneke, V. S. et al. Her2/neu testing in gastric cancer: evaluating the risk of sampling errors. Ann. Oncol. 24, 725–733 (2013).
Katai, H. et al. HER2 expression in carcinomas of the true cardia (Siewert type II esophagogastric junction carcinoma). World J. Surg. 38, 426–430 (2014).
Nagatsuma, A. K. et al. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer 18, 227–238 (2015).
Tanner, M. et al. Amplification of HER-2 in gastric carcinoma: association with topoisomerase IIα gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann. Oncol. 16, 273–278 (2005).
Janjigian, Y. Y. et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann. Oncol. 23, 2656–2662 (2012).
Terashima, M. et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin. Cancer Res. 18, 5992–6000 (2012).
Aizawa, M. et al. Evaluation of HER2-based biology in 1,006 cases of gastric cancer in a Japanese population. Gastric Cancer 17, 34–42 (2014).
Okines, A. F. et al. Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann. Oncol. 24, 1253–1261 (2013).
Wang, T. et al. Matched biopsy and resection specimens of gastric and gastroesophageal adenocarcinoma show high concordance in HER2 status. Hum. Pathol. 45, 970–975 (2014).
Gómez-Martín, C. et al. Consensus of the Spanish Society of Medical Oncology (SEOM) and Spanish Society of Pathology (SEAP) for HER2 testing in gastric carcinoma. Clin. Transl. Oncol. 13, 636–651 (2011).
Lordick, F. HER2 in gastric cancer: a biomarker with clinical impact, but not without translational challenges. Clin. Transl. Oncol. 13, 597–598 (2011).
Gullo, I. et al. Minimum biopsy set for HER2 evaluation in gastric and gastro-esophageal junction cancer. Endosc. Int. Open 3, E165–E170 (2015).
Tominaga, N. et al. Five biopsy specimens from the proximal part of the tumor reliably determine HER2 protein expression status in gastric cancer. Gastric Cancer http://dx.doi.org/10.1007/s10120-015-0502-3 (2015).
Kushima, R. et al. Interpretation of HER2 tests in gastric cancer: confirmation of interobserver differences and validation of a QA/QC educational program. Virchows Arch. 464, 539–545 (2014).
Gomez-Martin, C. et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J. Clin. Oncol. 31, 4445–4452 (2013).
Rüschoff, J. et al. HER2 testing in gastric cancer: a practical approach. Mod. Pathol. 25, 637–650 (2012).
Wainberg, Z. A. et al. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin. Cancer Res. 16, 1509–1519 (2010).
Satoh, T. et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN — a randomized, phase III study. J. Clin. Oncol. 32, 2039–2049 (2014).
Hecht, J. R. et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC — a randomized phase III Trial. J. Clin. Oncol. http://dx.doi.org/10.1200/JCO.2015.62.6598 (2015).
Lorenzen, S. et al. Lapatinib versus lapatinib plus capecitabine as second-line treatment in human epidermal growth factor receptor 2-amplified metastatic gastro-oesophageal cancer: a randomised phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Eur. J. Cancer 51, 569–576 (2015).
Blackwell, K. L. et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J. Clin. Oncol. 30, 2585–2592 (2012).
Yamashita-Kashima, Y. et al. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin. Cancer Res. 17, 5060–5070 (2011).
Kang, Y. K. et al. A phase IIa dose-finding and safety study of first-line pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2-positive advanced gastric cancer. Br. J. Cancer 111, 660–666 (2014).
Tabernero, J. et al. Pertuzumab (P) with trastuzumab (T) and chemotherapy (CTX) in patients (pts) with HER2-positive metastatic gastric or gastroesophageal junction (GEJ) cancer: an international phase III study (JACOB) [abstract]. J. Clin. Oncol. 31 (Suppl.), TPS4150 (2013).
Kang, Y. K. et al. A randomized, open-label, multicenter, adaptive phase 2/3 study of trastuzumab emtansine (T-DM1) versus a taxane (TAX) in patients (pts) with previously treated HER2-positive locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma (LA/MGC/GEJC) [abstract]. J. Clin. Oncol. 34 (Suppl. 4s), 5 (2016).
Rivera, F. et al. NeoHx study: perioperative treatment with trastuzumab in combination with capecitabine and oxaliplatin (XELOX-T) in patients with HER2 resectable stomach or esophagogastric junction (EGJ) adenocarcinoma — R0 resection, pCR, and toxicity analysis [abstract]. J. Clin. Oncol. 31 (Suppl.), 4098, (2013).
Hofheinz, R. D. et al. HER-FLOT: trastuzumab in combination with FLOT as perioperative treatment for patients with HER2-positive locally advanced esophagogastric adenocarcinoma: a phase II trial of the AIO Gastric Cancer Study Group [abstract]. J. Clin. Oncol. 32 (Suppl.), 4073 (2014).
U.S. National Library of Science. ClinicalTrials.gov [online], (2015).
U.S. National Library of Science. ClinicalTrials.gov [online], (2015).
Japan Clinical Oncology Group. A randomized phase II study of sysemic chemotherapy with and without trastuzumab followed by surgery in HER2 positive advanced gastric or esophagogastric junction adenocarcinoma with extensive lymph node metastasis (Trastuzumab In Gastric or Esophagogastric junction Adenocarcinoma): trigger study. [online],
GBG GERMAN BREAST GROUP et al. Trastuzumab improves the efficacy of chemotherapy in breast cancer treatment beyond progression. Breast Care (Basel) 3, 364–365 (2008).
Jackisch, C. et al. Impact of trastuzumab treatment beyond disease progression for advanced/metastatic breast cancer on survival — results from a prospective, observational study in Germany. Breast 23, 603–608 (2014).
Arteaga, C. L. & Engelman, J. A. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 25, 282–303 (2014).
Janjigian, Y. Y. et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in HER2-overexpressing esophagogastric (EG) tumors treated with trastuzumab. J. Clin. Oncol. 33 (Suppl. 3), 63 (2015).
Leyland-Jones, B. et al. Intensive loading dose of trastuzumab achieves higher-than-steady-state serum concentrations and is well tolerated. J. Clin. Oncol. 28, 960–966 (2010).
Oude Munnink, T. H. et al. Trastuzumab pharmacokinetics influenced by extent human epidermal growth factor receptor 2-positive tumor load. J. Clin. Oncol. 28, e355–e356; author reply e357 (2010).
Dijkers, E. C. et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 87, 586–592 (2010).
Oude Munnink, T. H. et al. Trastuzumab pharmacokinetics influenced by extent human epidermal growth factor receptor 2-positive tumor load. J. Clin. Oncol. 28, e355–e356 (2010).
Janjigian, Y. Y. et al. Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and 89Zr-trastuzumab PET. J. Nucl. Med. 54, 936–943 (2013).
Lordick, F. et al. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br. J. Cancer 102, 500–505 (2010).
Luber, B. et al. Biomarker analysis of cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric and oesophago-gastric junction cancer: results from a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie (AIO). BMC Cancer 11, 509 (2011).
Lordick, F. et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 14, 490–499 (2013).
Waddell, T. et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 14, 481–489 (2013).
Dragovich, T. et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J. Clin. Oncol. 24, 4922–4927 (2006).
Dutton, S. J. et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 15, 894–904 (2014).
Petty, R. D. et al. Epidermal growth factor receptor copy number gain (EGFR CNG) and response to gefitinib in esophageal cancer (EC): results of a biomarker analysis of a phase III trial of gefitinib versus placebo (TRANS-COG) [abstract]. J. Clin. Oncol. 32 (Suppl.), 4016 (2014).
Deng, N. et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 61, 673–684 (2012).
Lennerz, J. K. et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J. Clin. Oncol. 29, 4803–4810 (2011).
Iveson, T. et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 15, 1007–1018 (2014).
Lordick, F. Targeting the HGF/MET pathway in gastric cancer. Lancet Oncol. 15, 914–916 (2014).
Cunningham, D. et al. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study [abstract]. J. Clin. Oncol. 33 (Suppl.), 4000 (2015).
Shah, M. et al. METGastric: a phase III study of onartuzumab plus mFOLFOX6 in patients with metastatic HER2-negative (HER2-) and MET-positive (MET+) adenocarcinoma of the stomach or gastroesophageal junction (GEC) [abstract]. J. Clin. Oncol. 33 (Suppl.), 4012 (2015).
Kawakami, H. et al. MET amplification as a potential therapeutic target in gastric cancer. Oncotarget 4, 9–17 (2013).
Kwak, E. L. et al. Clinical activity of AMG 337, an oral MET kinase inhibitor, in adult patients (pts) with MET-amplified gastroesophageal junction (GEJ), gastric (G), or esophageal (E) cancer [abstract]. J. Clin. Oncol. 33 (Suppl. 3), 01 (2015).
Xie, L. et al. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin. Cancer Res. 19, 2572–2583 (2013).
Bang, J. Y. et al. A randomized, open-label phase II study of AZD4547 (AZD) versus paclitaxel (P) in previously treated patients with advanced gastric cancer (AGC) with fibroblast growth factor receptor 2 (FGFR2) polysomy or gene amplification (amp): SHINE study [abstract]. J. Clin. Oncol. 33 (Suppl.), 4014 (2015).
Smyth, E. C. et al. Phase II multicenter proof of concept study of AZD4547 in FGFR amplified tumours [abstract]. J. Clin. Oncol. 33 (Suppl.), 2508 (2015).
Ohtsu, A. et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J. Clin. Oncol. 31, 3935–3943 (2013).
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Clarke, J. M. & Hurwitz, H. I. Targeted inhibition of VEGF receptor 2: an update on ramucirumab. Expert Opin. Biol. Ther. 13, 1187–1196 (2013).
Thuss-Patience, P. C. et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer — a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur. J. Cancer 47, 2306–2314 (2011).
Kang, J. H. et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J. Clin. Oncol. 30, 1513–1518 (2012).
Ford, H. E. et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 15, 78–86 (2014).
Hironaka, S. et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J. Clin. Oncol. 31, 4438–4444 (2013).
Li, J. et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J. Clin. Oncol. 31, 3219–3225 (2013).
Anastassiadis, T. et al. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat. Biotechnol. 29, 1039–1045 (2011).
Wilhelm, S. et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 5, 835–844 (2006).
Janjigian, Y. Y. et al. Phase II trial of sorafenib in patients with chemotherapy refractory metastatic esophageal and gastroesophageal (GE) junction cancer. PLoS ONE 10, e0134731 (2015).
Pavlakis, N. et al. INTEGRATE: a randomized, phase II, double-blind, placebo-controlled study of regorafenib in refractory advanced oesophagogastric cancer (AOGC): a study by the Australasian Gastrointestinal Trials Group (AGITG) — final overall and subgroup results [abstract]. J. Clin. Oncol. 33 (Suppl.), 4003 (2015).
Ohtsu, A. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J. Clin. Oncol. 29, 3968–3976 (2011).
Shen, L. et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 18, 168–176 (2015).
Yoon, H. H. et al. Ramucirumab (RAM) plus FOLFOX as front-line therapy (Rx) for advanced gastric or esophageal adenocarcinoma (GE-AC): randomized, double-blind, multicenter phase 2 trial [abstract]. J. Clin. Oncol. 32 (Suppl.), 4004 (2014).
Erber, R. et al. Combined inhibition of VEGF- and PDGF-signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 18, 338–340 (2004).
Fischer, C. et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131, 463–475 (2007).
Fuchs, C. S. et al. A randomized, double-blind, placebo-controlled phase III study of cisplatin plus a fluoropyrimidine with or without ramucirumab as first-line therapy in patients with metastatic gastric or gastroesophogeal junction (GEJ) adenocarcinoma (RAINFALL, NCT02314117) [abstract]. J. Clin. Oncol. 33 (Suppl.), TPS4131 (2015).
Van Cutsem, E. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol. 30, 2119–2127 (2012).
Lapidot, T. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 17, 645–648 (1994).
Kaiser, J. The cancer stem cell gamble. Science 347, 226–229 (2015).
Patrawala, L. et al. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 65, 6207–6219 (2005).
Patrawala, L. et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 25, 1696–1708 (2006).
Vermeulen, L. et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12, 468–476 (2010).
Vries, R. G. et al. Stem cells and cancer of the stomach and intestine. Mol. Oncol. 4, 373–384 (2010).
Marx, J. Cancer's perpetual source? Science 317, 1029–1031 (2007).
Scheitz, C. J. et al. Defining a tissue stem cell-driven Runx1/Stat3 signalling axis in epithelial cancer. EMBO J. 31, 4124–4139 (2012).
Yu, H. et al. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat. Rev. Cancer 14, 736–746 (2014).
Susman, S. et al. The Lauren classification highlights the role of epithelial-to-mesenchymal transition in gastric carcinogenesis: an immunohistochemistry study of the STAT3 and adhesion molecules expression. J. Gastrointestin. Liver Dis. 24, 77–83 (2015).
Li, Y. et al. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl Acad. Sci. USA 112, 1839–1844 (2015).
Shah, M. A. et al. The BRIGHTER trial: a phase III randomized double-blind study of BBI608 + weekly paclitaxel versus placebo (PBO) + weekly paclitaxel in patients (pts) with pretreated advanced gastric and gastro-esophageal junction (GEJ) adenocarcinoma [abstract]. J. Clin. Oncol. 33 (Suppl.), PS4139 (2015).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
Lesokhin, A. M. et al. On being less tolerant: enhanced cancer immunosurveillance enabled by targeting checkpoints and agonists of T cell activation. Sci. Transl. Med. 7, 280sr1 (2015).
Doi, T. et al. Pembrolizumab (MK-3475) for patients (pts) with advanced esophageal carcinoma: preliminary results from KEYNOTE-028 [abstract]. J. Clin. Oncol. 33 (Suppl.), 4010 (2015).
Muro, K. et al. Relationship between PD-L1 expression and clinical outcomes in patients (Pts) with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab (Pembro; MK-3475) in KEYNOTE-012 [abstract]. J. Clin. Oncol. 33 (Suppl. 3), 03 (2015).
Curran, M. A. et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl Acad. Sci. USA 107, 4275–4280 (2010).
Duraiswamy, J. et al. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 369, 122–133 (2013).
Callahan, M. et al. Phase I/II, open-label study of nivolumab (anti-PD-1; BMS-936558, ONO-4538) as monotherapy or combined with ipilimumab advanced or metastatic solid tumor [abstract]. J. Clin. Oncol. 32 (Suppl.), TPS3114 (2014).
Tran, E. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645 (2014).
Papa, S. et al. Clinical evaluation of ErbB-targeted CAR T-cells, following intracavity delivery in patients with ErbB-expressing solid tumors. Methods Mol. Biol. 1317, 365–382 (2015).
Ahmed, N. et al. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2- positive sarcoma. J. Clin. Oncol. 33, 1688–1696 (2015).
Lin, S. J. et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut 64, 1721–1731 (2015).
Author information
Authors and Affiliations
Contributions
Both authors made substantial contributions to researching data for article, discussion of content, and review/editing of manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
F.L. has lectured and chaired sponsored symposia for Amgen, Celgene, Eli Lilly, Elsevier, Roche and Taiho. He has received support for participation in scientific congresses from Amgen, Bayer, Eli Lilly, Merck-Serono, Roche and Taiho. He receives research support from Fresenius Biotech, GSK and Merck-Serono. He has also served on advisory boards for BMS, Eli Lilly, Nordic, Roche and Taiho. Y.J. has received research funding from Amgen, Bayer, Boehringer Ingelheim, Eli Lilly, Genentech and Merck. She has also served on advisory boards for Eli Lilly and Pfizer.
Rights and permissions
About this article
Cite this article
Lordick, F., Janjigian, Y. Clinical impact of tumour biology in the management of gastroesophageal cancer. Nat Rev Clin Oncol 13, 348–360 (2016). https://doi.org/10.1038/nrclinonc.2016.15
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.15
This article is cited by
-
Efficacy of PD-1/PD-L1 inhibitors in gastric or gastro-oesophageal junction cancer based on clinical characteristics: a meta-analysis
BMC Cancer (2023)
-
Wnt/β-catenin pathway is a key signaling pathway to trastuzumab resistance in gastric cancer cells
BMC Cancer (2023)
-
Intratumour Fusobacterium nucleatum and immune response to oesophageal cancer
British Journal of Cancer (2023)
-
Single-cell analysis of gastric signet ring cell carcinoma reveals cytological and immune microenvironment features
Nature Communications (2023)
-
Deviating HER2 test results in gastric cancer: analysis from the prospective multicenter VARIANZ study
Journal of Cancer Research and Clinical Oncology (2023)