Key Points
-
Cancer cells leaving the immunosuppressive microenvironment of the primary tumour become vulnerable to immune surveillance and require mechanisms of escape from immune-mediated elimination if they are to form metastases
-
Circulating tumour cells (CTCs) and disseminated tumour cells (DTCs) are often detectable in the peripheral blood and bone marrow, respectively, of patients with any of a range of different malignancies
-
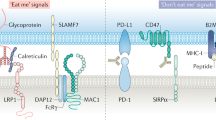
CTCs and DTCs exploit a large variety of immune-escape mechanisms, including alterations in the expression of MHC molecules, NK-cell ligands, FAS, FAS ligand (FASL), and immune-checkpoint molecules, such as CD47 and programmed cell death 1 ligand 1 (PD-L1)
-
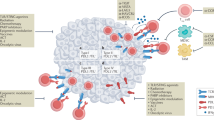
CTC homing to distant organs can be supported by direct interactions with immune cells during the process of extravasation, and by the effects of inflammatory cytokines in the target organ
-
Future studies must address the important question of how the immune system shapes the molecular composition of CTCs and DTCs during cancer dormancy and metastatic progression
Abstract
Metastatic spread of tumour cells is the main cause of cancer-related deaths. Understanding the mechanisms of tumour-cell dissemination has, therefore, become an important focus for cancer research. In patients with cancer, disseminated cancer cells are often detectable in the peripheral blood as circulating tumour cells (CTCs) and in the bone marrow or lymph nodes as disseminated tumour cells (DTCs). The identification and characterization of CTCs and DTCs has yielded important insights into the mechanisms of metastasis, resulting in a better understanding of the molecular alterations and profiles underlying drug resistance. Given the expanding role of immunotherapies in the treatment of cancer, interactions between tumour cells and immune cells are the subject of intense research. Theoretically, cancer cells that exit the primary tumour site — leaving the protection of the typically immunosuppressive tumour microenvironment — will be more vulnerable to attack by immune effector cells; thus, the survival of tumour cells after dissemination might be the 'Achilles' heel' of metastatic progression. In this Review, we discuss findings relating to the interactions of CTCs and DTCs with the immune system, in the context of cancer immuno-editing, evasion from immune surveillance, and formation of metastases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Braun, S. et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J. Clin. Oncol. 18, 80–86 (2000).
Naume, B. et al. Clinical outcome with correlation to disseminated tumor cell (DTC) status after DTC-guided secondary adjuvant treatment with docetaxel in early breast cancer. J. Clin. Oncol. 32, 3848–3857 (2014).
Bidard, F.-C. et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 15, 406–414 (2014).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005).
Lokody, I. Cancer genetics: the origin and evolution of an ancient cancer. Nat. Rev. Cancer 14, 152 (2014).
Zhang, L. et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin. Cancer Res. 18, 5701–5710 (2012).
Jannasch, K. et al. Chemotherapy of WAP-T mouse mammary carcinomas aggravates tumor phenotype and enhances tumor cell dissemination. Int. J. Cancer 137, 25–36 (2015).
Juacaba, S. F., Horak, E., Price, J. E. & Tarin, D. Tumor cell dissemination patterns and metastasis of murine mammary carcinoma. Cancer Res. 49, 570–575 (1989).
Pantel, K. & Alix-Panabières, C. Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. Bonekey Rep. 3, 584 (2014).
Pantel, K., Brakenhoff, R. H. & Brandt, B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat. Rev. Cancer 8, 329–340 (2008).
Braun, S. & Naume, B. Circulating and disseminated tumor cells. J. Clin. Oncol. 23, 1623–1626 (2005).
Bidard, F. C. et al. Disseminated tumor cells and the risk of locoregional recurrence in nonmetastatic breast cancer. Ann. Oncol. 20, 1836–1841 (2009).
Kim, M. Y. et al. Tumor self-seeding by circulating cancer cells. Cell 139, 1315–1326 (2009).
Pagès, F. et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 29, 1093–1102 (2010).
Crespo, J., Sun, H., Welling, T. H., Tian, Z. & Zou, W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 25, 214–221 (2013).
Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
Muller, C. et al. Hematogenous dissemination of glioblastoma multiforme. Sci. Transl. Med. 6, 247ra101 (2014).
Macarthur, K. M. et al. Detection of brain tumor cells in the peripheral blood by a telomerase promoter-based assay. Cancer Res. 74, 2152–2159 (2014).
Sullivan, J. P. et al. Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discov. 11, 1299–1309 (2014).
Pietschmann, S. et al. An individual patient data meta-analysis on characteristics, treatments and outcomes of glioblastoma/gliosarcoma patients with metastases outside of the central nervous system. PLoS ONE 10, e0121592 (2015).
Burnet, F. M. The concept of immunological surveillance. Prog. Exp. Tumor Res. 13, 1–27 (1970).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360 (2004).
Corthay, A. Does the immune system naturally protect against cancer? Front. Immunol. 5, 197 (2014).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 (2002).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Khong, H. T. & Restifo, N. P. Natural selection of tumor variants in the generation of 'tumor escape' phenotypes. Nat. Immunol. 3, 999–1005 (2002).
Koebel, C. M. et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450, 903–907 (2007).
Teng, M. W. L., Swann, J. B., Koebel, C. M., Schreiber, R. D. & Smyth, M. J. Immune-mediated dormancy: an equilibrium with cancer. J. Leukoc. Biol. 84, 988–993 (2008).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Dunn, G. P., Koebel, C. M. & Schreiber, R. D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6, 836–848 (2006).
Dighe, A. S., Richards, E., Old, L. J. & Schreiber, R. D. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFNγ receptors. Immunity 1, 447–456 (1994).
Ribas, A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 5, 915–919 (2015).
Oleinika, K., Nibbs, R. J., Graham, G. J. & Fraser, A. R. Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin. Exp. Immunol. 171, 36–45 (2013).
Facciabene, A., Motz, G. T. & Coukos, G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 72, 2162–2171 (2012).
Wu, A. A., Drake, V., Huang, H.-S., Chiu, S. & Zheng, L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology 4, e1016700 (2015).
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293–301 (2015).
Waldhauer, I. & Steinle, A. NK cells and cancer immunosurveillance. Oncogene 27, 5932–5943 (2008).
Pantel, K. et al. Frequent down-regulation of major histocompatibility class I antigen expression on individual micrometastatic carcinoma cells. Cancer Res. 51, 4712–4715 (1991).
Green, T. L., Cruse, J. M., Lewis, R. E. & Craft, B. S. Circulating tumor cells (CTCs) from metastatic breast cancer patients linked to decreased immune function and response to treatment. Exp. Mol. Pathol. 95, 174–179 (2013).
Santos, M. F. et al. Comparative analysis of innate immune system function in metastatic breast, colorectal, and prostate cancer patients with circulating tumor cells. Exp. Mol. Pathol. 96, 367–374 (2014).
Jewett, A. & Tseng, H. Tumor induced inactivation of natural killer cell cytotoxic function; implication in growth, expansion and differentiation of cancer stem cells. J. Cancer 2, 443–457 (2011).
Paolino, M. et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature 507, 508–512 (2014).
Green, T. L. et al. Toll-like receptor (TLR) expression of immune system cells from metastatic breast cancer patients with circulating tumor cells. Exp. Mol. Pathol. 97, 44–48 (2014).
Bellora, F. et al. TLR activation of tumor-associated macrophages from ovarian cancer patients triggers cytolytic activity of NK cells. Eur. J. Immunol. 44, 1814–1822 (2014).
Biswas, S. K. & Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 11, 889–896 (2010).
Adib-Conquy, M., Scott-Algara, D., Cavaillon, J.-M. & Souza-Fonseca-Guimaraes, F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol. Cell Biol. 92, 256–262 (2014).
Hanna, N. Role of natural killer cells in control of cancer metastasis. Cancer Metastasis Rev. 1, 45–64 (1982).
Brodbeck, T., Nehmann, N., Bethge, A., Wedemann, G. & Schumacher, U. Perforin-dependent direct cytotoxicity in natural killer cells induces considerable knockdown of spontaneous lung metastases and computer modelling-proven tumor cell dormancy in a HT29 human colon cancer xenograft mouse model. Mol. Cancer 13, 244 (2014).
Takeda, K. et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat. Med. 7, 94–100 (2001).
Mitchell, M. J., Wayne, E., Rana, K., Schaffer, C. B. & King, M. R. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc. Natl Acad. Sci. USA 111, 930–935 (2014).
Gül, N. et al. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J. Clin. Invest. 124, 812–823 (2014).
Gül, N., Babes, L., Kubes, P. & van Egmond, M. Macrophages in the liver prevent metastasis by efficiently eliminating circulating tumor cells after monoclonal antibody immunotherapy. Oncoimmunology 3, e28441 (2014).
Bayon, L., Izquierdo, M. & Sirovich, I. Role of Kupffer cells in arresting circulating tumor cells and controlling metastatic growth in the liver. Hepatology 23, 1224–1231 (1996).
Denève, E. et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin. Chem. 59, 1384–1392 (2013).
Van den Eynden, G. G. et al. The multifaceted role of the microenvironment in liver metastasis: biology and clinical implications. Cancer Res. 73, 2031–2043 (2013).
Galon, J., Costes, A. & Sanchez-Cabo, F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1965 (2006).
Galon, J., Angell, H. K., Bedognetti, D. & Marincola, F. M. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 39, 11–26 (2013).
Fridman, W. H. et al. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 71, 5601–5605 (2011).
Shankaran, V., Ikeda, H. & Bruce, A. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001).
De Giorgi, U. et al. Relationship between lymphocytopenia and circulating tumor cells as prognostic factors for overall survival in metastatic breast cancer. Clin. Breast Cancer 12, 264–269 (2012).
Gruber, I. et al. Relationship between circulating tumor cells and peripheral T-cells in patients with primary breast cancer. Anticancer Res. 33, 2233–2238 (2013).
Mego, M. et al. Circulating tumor cells (CTC) are associated with defects in adaptive immunity in patients with inflammatory breast cancer. J. Cancer 7, 1095–1104 (2016).
Chang, Y. S. et al. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc. Natl Acad. Sci. USA 97, 14608–14613 (2000).
Dougan, M. & Dranoff, G. in Current Protocols in Immunology. Ch. 20, Unit 20.11 (ed. Coico, R.) (Wiley, 2009).
Aptsiauri, N. et al. Role of altered expression of HLA class I molecules in cancer progression. Adv. Exp. Med. Biol. 601, 123–131 (2007).
Placke, T. et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 72, 440–448 (2012).
Gay, L. & Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 11, 123–134 (2011).
Labelle, M., Begum, S. & Hynes, R. O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20, 576–590 (2011).
Palumbo, J. S. et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell – mediated elimination of tumor cells. Blood 105, 178–186 (2014).
Camerer, E. et al. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 104, 397–401 (2004).
Leblanc, R. & Peyruchaud, O. Metastasis: new functional implications of platelets and megakaryocytes. Blood 128, 24–31 (2016).
Wu, M.-S., Li, C.-H., Ruppert, J. G. & Chang, C.-C. Cytokeratin 8–MHC class I interactions: a potential novel immune escape phenotype by a lymph node metastatic carcinoma cell line. Biochem. Biophys. Res. Commun. 441, 618–623 (2013).
Moll, R., Franke, W., Schiller, D., Geiger, B. & Krepler, R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31, 11–24 (1982).
Nausch, N. & Cerwenka, A. NKG2D ligands in tumor immunity. Oncogene 27, 5944–5958 (2008).
Wang, B. et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 74, 5746–5757 (2014).
Barsoum, I. B. et al. Hypoxia induces escape from innate immunity in cancer cells via increased expression of ADAM10: role of nitric oxide. Cancer Res. 71, 7433–7441 (2011).
Crane, C. A. et al. Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc. Natl Acad. Sci. USA 5, 12823–12828 (2014).
Deng, W. et al. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science 348, 136–139 (2015).
Kochan, G., Escors, D., Breckpot, K. & Guerrero-Setas, D. Role of non-classical MHC class I molecules in cancer immunosuppression. Oncoimmunology 2, e26491 (2013).
Lin, A. & Yan, W.-H. HLA-G expression in cancers: roles in immune evasion, metastasis and target for therapy. Mol. Med. 21, 782–791 (2015).
He, X. et al. HLA-G expression in human breast cancer: implications for diagnosis and prognosis, and effect on allocytotoxic lymphocyte response after hormone treatment in vitro. Ann. Surg. Oncol. 17, 1459–1469 (2010).
de Kruijf, E. M. et al. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J. Immunol. 185, 7452–7459 (2010).
Guo, Z.-Y. et al. Predictive value of HLA-G and HLA-E in the prognosis of colorectal cancer patients. Cell. Immunol. 293, 10–16 (2015).
Cai, M.-Y. et al. Human leukocyte antigen-G protein expression is an unfavorable prognostic predictor of hepatocellular carcinoma following curative resection. Clin. Cancer Res. 15, 4686–4693 (2009).
Wiendl, H. et al. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J. Immunol. 168, 4772–4780 (2002).
Loumagne, L. et al. In vivo evidence that secretion of HLA-G by immunogenic tumor cells allows their evasion from immunosurveillance. Int. J. Cancer 135, 2107–2117 (2014).
Agaugué, S., Carosella, E. D. & Rouas-Freiss, N. Role of HLA-G in tumor escape through expansion of myeloid-derived suppressor cells and cytokinic balance in favor of Th2 versus Th1/Th17. Blood 117, 7021–7031 (2011).
Contini, P. et al. Soluble HLA-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur. J. Immunol. 33, 125–134 (2003).
König, L. et al. The prognostic impact of soluble and vesicular HLA-G and its relationship to circulating tumor cells in neoadjuvant treated breast cancer patients. Hum. Immunol. 77, 791–799 (2016).
Owen-Schaub, L., Chan, H., Cusack, J. C., Roth, J. & Hill, L. L. Fas and Fas ligand interactions in malignant disease. Int. J. Oncol. 17, 5–12 (2000).
Terheyden, P. et al. Predominant expression of Fas (CD95) ligand in metastatic melanoma revealed by longitudinal analysis. J. Invest. Dermatol. 112, 899–902 (1999).
Nozoe, T., Yasuda, M., Honda, M., Inutsuka, S. & Korenaga, D. Fas ligand expression is correlated with metastasis in colorectal carcinoma. Oncology 65, 83–88 (2003).
Gutierrez, L. S., Eliza, M., Niven-Fairchild, T., Naftolin, F. & Mor, G. The Fas/Fas-ligand system: a mechanism for immune evasion in human breast carcinomas. Breast Cancer Res. Treat. 54, 245–253 (1999).
Gordon, N. & Kleinerman, E. S. The role of Fas/FasL in the metastatic potential of osteosarcoma and targeting this pathway for the treatment of osteosarcoma lung metastases. Cancer Treat. Res. 152, 497–508 (2009).
Owen-Schaub, L. B., van Golen, K. L., Hill, L. L. & Price, J. E. Fas and Fas ligand interactions suppress melanoma lung metastasis. J. Exp. Med. 188, 1717–1723 (1998).
Strand, S. et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells — a mechanism of immune evasion? Nat. Med. 2, 1361–1366 (1996).
Gruber, I. V. et al. Down-regulation of CD28, TCR-zeta (ζ) and up-regulation of FAS in peripheral cytotoxic T-cells of primary breast cancer patients. Anticancer Res. 28, 779–784 (2008).
Strauss, L., Bergmann, C. & Whiteside, T. L. Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J. Immunol. 182, 1469–1480 (2009).
Ugurel, S., Rappl, G., Tilgen, W. & Reinhold, U. Increased soluble CD95 (sFas/CD95) serum level correlates with poor prognosis in melanoma patients. Clin. Cancer Res. 7, 1282–1286 (2001).
Cheng, J. et al. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 263, 1759–1762 (1994).
Hallermalm, K., De Geer, A., Kiessling, R., Levitsky, V. & Levitskaya, J. Autocrine secretion of Fas ligand shields tumor cells from Fas-mediated killing by cytotoxic lymphocytes. Cancer Res. 64, 6775–6782 (2004).
Kim, R., Emi, M., Tanabe, K. & Arihiro, K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 66, 5527–5536 (2006).
Chao, M. P., Majeti, R. & Weissman, I. L. Programmed cell removal: a new obstacle in the road to developing cancer. Nat. Rev. Cancer 12, 58–67 (2011).
Jaiswal, S. et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138, 271–285 (2009).
Steinert, G. et al. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 74, 1694–1704 (2014).
Chao, M. P. et al. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood 118, 4890–4901 (2011).
Baccelli, I. et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 31, 539–544 (2013).
Baccelli, I. et al. Co-expression of MET and CD47 is a novel prognosticator for survival of luminal-type breast cancer patients. Oncotarget 5, 8147–8160 (2014).
Noman, M. Z., Messai, Y., Muret, J., Hasmim, M. & Chouaib, S. Crosstalk between CTC, immune system and hypoxic tumor microenvironment. Cancer Microenviron. 7, 153–160 (2014).
Kallergi, G. et al. Hypoxia-inducible factor-α and vascular endothelial growth factor expression in circulating tumor cells of breast cancer patients. Breast Cancer Res. 11, R84 (2009).
Bartkowiak, K., Riethdorf, S. & Pantel, K. The interrelating dynamics of hypoxic tumor microenvironments and cancer cell phenotypes in cancer metastasis. Cancer Microenviron. 5, 59–72 (2012).
Bartkowiak, K. et al. Disseminated tumor cells persist in the bone marrow of breast cancer patients through sustained activation of the unfolded protein response. Cancer Res. 75, 5367–5377 (2015).
Barrett, D. M., Singh, N., Porter, D. L., Grupp, S. A. & June, C. H. Chimeric antigen receptor therapy for cancer. Annu. Rev. Med. 65, 333–347 (2014).
Sharma, P. The future of immune checkpoint therapy. Science 348, 56–61 (2014).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Zou, W. & Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 8, 467–477 (2008).
Mego, M. et al. Prognostic value of EMT-circulating tumor cells in metastatic breast cancer patients undergoing high-dose chemotherapy with autologous hematopoietic stem cell transplantation. J. Cancer 3, 369–380 (2012).
Joosse, S. A., Gorges, T. M. & Pantel, K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 7, 1–11 (2014).
Alsuliman, A. et al. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Mol. Cancer 14, 149 (2015).
Chen, L. et al. Metastasis is regulated via microRNA- 200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 5, 5241 (2014).
Wang, Y. et al. PD-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma. Med. Oncol. 32, 212 (2015).
Ock, C.-Y. et al. PD-L1 expression is associated with epithelial–mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget 7, 15901–15914 (2016).
Lee, Y. et al. CD44+ cells in head and neck squamous cell carcinoma suppress T-cell-mediated immunity by selective constitutive and inducible expression of PD-L1. Clin. Cancer Res. 22, 3571–3581 (2016).
Cohen, E. N. et al. Inflammation mediated metastasis: immune induced epithelial-to-mesenchymal transition in inflammatory breast cancer cells. PLoS ONE 10, e0132710 (2015).
Mego, M. et al. CXCR4–SDF-1 interaction potentially mediates trafficking of circulating tumor cells in primary breast cancer. BMC Cancer 16, 127 (2016).
Shin, D. S. & Ribas, A. The evolution of checkpoint blockade as a cancer therapy: what's here, what's next? Curr. Opin. Immunol. 33, 23–35 (2015).
Romagné, F. et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 114, 2667–2677 (2009).
Benson, D. M. et al. A phase I trial of the anti-KIR antibody IPH2101 and lenalidomide in patients with relapsed/refractory multiple myeloma. Clin. Cancer Res. 21, 4055–4061 (2015).
Mazel, M. et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 9, 1773–1782 (2015).
David, R. PD-L1 expression by circulating breast cancer cells. Lancet Oncol. 16, e321 (2015).
Oliveira-Costa, J. P. et al. Gene expression patterns through oral squamous cell carcinoma development: PD-L1 expression in primary tumor and circulating tumor cells. Oncotarget 6, 20902–20920 (2015).
Jakóbisiak, M., Lasek, W. & Golb, J. Natural mechanisms protecting against cancer. Immunol. Lett. 90, 103–122 (2003).
Smith, H. A. & Kang, Y. The metastasis-promoting roles of tumor-associated immune cells. J. Mol. Med. 91, 411–429 (2013).
Kitamura, T., Qian, B.-Z. & Pollard, J. W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 15, 73–86 (2015).
Wolf, A. M. et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin. Cancer Res. 9, 606–612 (2003).
Jiang, H. et al. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int. J. Cancer 136, 2352–2360 (2014).
Wilke, C. M., Wu, K., Zhao, E., Wang, G. & Zou, W. Prognostic significance of regulatory T cells in tumor. Int. J. Cancer 127, 748–758 (2010).
Cole, S. et al. Elevated circulating myeloid derived suppressor cells (MDSC) are associated with inferior overall survival (OS) and correlate with circulating tumor cells (CTC) in patients with metastatic breast cancer. Cancer Res. 69, 4135–4135 (2009).
Stanzer, S. et al. Resistance to apoptosis and expansion of regulatory T cells in relation to the detection of circulating tumor cells in patients with metastatic epithelial cancer. J. Clin. Immunol. 28, 107–114 (2008).
Aggouraki, D. et al. Correlation of circulating tumor cells (CTCs) expressing stemness and EMT phenotypes with immunosuppressive cells in metastatic breast cancer patients. Cancer Res. 74, abstr. 3030 (2014).
Hensler, M. et al. Gene expression profiling of circulating tumor cells and peripheral blood mononuclear cells from breast cancer patients. Oncoimmunology 5, e1102827 (2016).
Zhang, P. et al. PARP-1 controls immunosuppressive function of regulatory T cells by destabilizing Foxp3. PLoS ONE 8, e71590 (2013).
Taranova, A. G. et al. Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Res. 68, 8582–8589 (2008).
Tseng, J.-Y. et al. Interleukin-17A modulates circulating tumor cells in tumor draining vein of colorectal cancers and affects metastases. Clin. Cancer Res. 20, 2885–2897 (2014).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–1375 (2006).
Connelly, L. et al. NF-κB activation within macrophages leads to an anti-tumor phenotype in a mammary tumor lung metastasis model. Breast Cancer Res. 13, R83 (2011).
Jacobs, P. P. & Sackstein, R. CD44 and HCELL: preventing hematogenous metastasis at step 1. FEBS Lett. 585, 3148–3158 (2011).
Gakhar, G. et al. Circulating tumor cells from prostate cancer patients interact with E-selectin under physiologic blood flow. PLoS ONE 8, e85143 (2013).
Lee, N., Barthel, S. R. & Schatton, T. Melanoma stem cells and metastasis: mimicking hematopoietic cell trafficking? Lab. Invest. 94, 13–30 (2014).
McDonald, B. et al. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int. J. Cancer 125, 1298–1305 (2009).
Kate, M. Ten & Aalbers, A. Polymorphonuclear leukocytes increase the adhesion of circulating tumor cells to microvascular endothelium. Anticancer Res. 27, 17–22 (2007).
Spicer, J. D. et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 72, 3919–3927 (2012).
Strell, C., Lang, K., Niggemann, B., Zaenker, K. S. & Entschladen, F. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp. Cell Res. 316, 138–148 (2010).
Huh, S. J., Liang, S., Sharma, A., Dong, C. & Robertson, G. P. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 70, 6071–6082 (2010).
Dong, C. & Slattery, M. Melanoma cell extravasation under flow conditions is modulated by leukocytes and endogenously produced interleukin 8. Mol. Cell. Biochem. 2, 145–159 (2005).
Dimitroff, C. J., Lee, J. Y., Fuhlbrigge, R. C. & Sackstein, R. A distinct glycoform of CD44 is an L-selectin ligand on human hematopoietic cells. Proc. Natl Acad. Sci. USA 97, 13841–13846 (2000).
Hanley, W. D. et al. Variant isoforms of CD44 are P- and L-selectin ligands on colon carcinoma cells. FASEB J. 20, 337–339 (2006).
Demers, M. et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl Acad. Sci. USA 109, 13076–13081 (2012).
Cedervall, J. et al. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. 75, 2653–2662 (2015).
Alfaro, C. et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin. Cancer Res. 22, 3924–3936 (2016).
Cools-Lartigue, J. et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 123, 3446–3458 (2013).
Cayrefourcq, L. et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 75, 982–901 (2015).
Yu, M. et al. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345, 216–220 (2014).
Gates, J. D. et al. Monitoring circulating tumor cells in cancer vaccine trials. Hum. Vaccin. 4, 389–392 (2008).
Stojadinovic, A. et al. Quantification and phenotypic characterization of circulating tumor cells for monitoring response to a preventive HER2/neu vaccine-based immunotherapy for breast cancer: a pilot study. Ann. Surg. Oncol. 14, 3359–3368 (2007).
Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377 (2008).
Strati, A. et al. Gene expression profile of circulating tumor cells in breast cancer by RT-qPCR. BMC Cancer 11, 422 (2011).
Gasch, C. et al. Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. Clin. Chem. 59, 252–260 (2013).
Zaidi, M. R. & Merlino, G. The two faces of interferon-γ in cancer. Clin. Cancer Res. 17, 6118–6124 (2011).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Acknowledgements
We apologize to all authors who have published excellent work in this field that we could not cite in this concise Review. We also thank Prof. Wolfgang Deppert (Institute for Tumour Biology, University Medical Centre Hamburg-Eppendorf (UKE), Hamburg, Germany) and Dr Cécile Maire (Department of Neurosurgery, UKE) for critical reading of the manuscript, as well as Prof. Katrin Lamszus and Prof. Manfred Westphal (Department of Neurosurgery, UKE) for their continuous support of our work. The work of the authors has been supported by the European Research Council (ERC Advanced Investigator Grant DISSECT (to K.P.).
Author information
Authors and Affiliations
Contributions
Each author made substantial contributions to researching the data, discussions of content, writing, and review/editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Mohme, M., Riethdorf, S. & Pantel, K. Circulating and disseminated tumour cells — mechanisms of immune surveillance and escape. Nat Rev Clin Oncol 14, 155–167 (2017). https://doi.org/10.1038/nrclinonc.2016.144
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.144
This article is cited by
-
Genetically engineered membrane-based nanoengagers for immunotherapy of pancreatic cancer
Journal of Nanobiotechnology (2024)
-
Single-cell low-pass whole genome sequencing accurately detects circulating tumor cells for liquid biopsy-based multi-cancer diagnosis
npj Precision Oncology (2024)
-
Tumor heterogeneity and clinically invisible micrometastases in metastatic breast cancer—a call for enhanced surveillance strategies
npj Precision Oncology (2024)
-
Immune checkpoints HLA-E:CD94-NKG2A and HLA-C:KIR2DL1 complementarily shield circulating tumor cells from NK-mediated immune surveillance
Cell Discovery (2024)
-
Survival mechanisms of circulating tumor cells and their implications for cancer treatment
Cancer and Metastasis Reviews (2024)