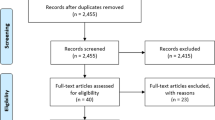
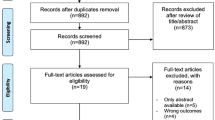
Abstract
Many novel therapies are available for use in patients with metastatic castration-resistant prostate cancer (mCRPC), some of which convey substantial progression-free survival and overall survival benefits. Delaying disease progression and providing palliation of symptoms are primary therapeutic aims of treating patients with mCRPC; therefore, ensuring that the benefit-to-harm ratios are acceptable to patients, through systematic measurement of patient-reported outcomes (PROs) using validated tools, is vital. In this Perspectives, we appraised the published reports from clinical trials testing treatments of mCRPC over the past 5 years and found that PROs were either not being measured routinely, or if used, were often not reported adequately, thus hampering evaluation of the true effects of many of these treatments on patients' quality of life. Improvements are needed because data collected directly from patients, not just physician-collected safety data and adverse events, are crucial to inform clinical decision-making on treatment options.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
[No authors listed.] Prostate cancer mortality in Europe and worldwide. Cancer Research UK http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/mortality#heading-Three (2016).
de Bono, J. S. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154 (2010).
Berthold, D. R. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J. Clin. Oncol. 26, 242–245 (2008).
Parker, C. C. et al. A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur. Urol. 63, 189–197 (2013).
Kantoff, P. & Higano, C. S. Integration of immunotherapy into the management of advanced prostate cancer. Urol. Oncol. 30, S41–S47 (2012).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Ryan, C. J. et al. Randomized phase 3 trial of abiraterone acetate in men with metastatic castration-resistant prostate cancer and no prior chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Beer, T. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Atkinson, T. M. et al. Reliability of adverse symptom event reporting by clinicians. Qual. Life Res. 21, 1159–1164 (2012).
Basch, E. 'The missing voice of patients in drug-safety reporting'. N. Engl. J. Med. 362, 865–869 (2010).
Turner, S. Maher, E. J. Young, T. Young, J. & Vaughan Hudson, G. What are the information priorities for cancer patients involved in treatment decisions? An experienced surrogate study in Hodgkin's disease. Br. J. Cancer 73, 222–227 (1996).
Bryant-Lukosius, D. et al. Evaluating health-related quality of life and priority health problems in patients with prostate cancer: a strategy for defining the role of the advanced practice nurse. Can. Oncol. Nurs. J. 20, 5–14 (2010).
Moul, J. W. & Dawson, N. Quality of life associated with treatment of castration resistant prostate cancer: a review opf the literature. Cancer Invest. 30, 1–12 (2012).
Ellis, L. M et al. American Society of Clinical Oncology perspective: raising the bar for clinical trials by defining clinically meaningful outcomes. 32, 1277–1280 (2014).
Cherny, N. I. et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann. Oncol. 26, 1547–1573 (2015).
US Department of Health and Human Services. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labelling claims. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf (2009).
European Medicines Agency, Committee for Medicinal Products for Human Use. Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man. The use of patient-reported outcome (PRO) measures in oncology studies. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2016/04/WC500205159.pdf (2016).
Vodicka, E. et al. Inclusion of patient-reported outcome measures in registered clinical trials: evidence from ClinicalTrials. gov (2007–2013). Contemp. Clin. Trials 43, 1–9 (2015).
Calvert, M. et al. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 309, 814–822 (2013).
Scher, H. et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J. Clin. Oncol. 34, 1402–1418 (2016).
Fizazi, K. et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 15, 1147–1156 (2014).
Cella, D. et al. Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Ann. Oncol. 26, 179–185 (2015).
Loriot, Y. et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 16, 509–521 (2015).
Logothetis, C. J. et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 13, 1210–1217 (2012).
Harland, S. et al. Effect of abiraterone acetate treatment on the quality of life of patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy. Eur. J. Cancer 49, 3648–3657 (2013).
Sternberg, C. N. et al. Effect of abiraterone acetate on fatigue in patients with metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Ann. Oncol. 24, 1017–1025 (2013).
Basch, E. et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol. 14, 1193–1199 (2013).
Caffo, O. et al. Impact of docetaxel-based chemotherapy on quality of life of patients with castration resistant prostate cancer: results from a prospective phase II randomized trial. BJU Int. 108, 1825–1832 (2011).
Caffo, O. et al. Intermittent docetaxel chemotherapy as first-line treatment for metastatic castration-resistant prostate cancer patients. Future Oncol. 11, 1845–1845 (2015).
Bahl, A. et al. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann. Oncol. 24, 2402–2408 (2013).
Bahl, A. et al. Final quality of life and safety data for patients with metastatic castration-resistant prostate cancer treated with cabazitaxel in the UK Early Access Programme (EAP) (NCT01254279). BJU Int. 116, 880–887 (2015).
Nilsson, S. et al. A randomized, dose–response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur. J. Cancer 48, 678–686 (2012).
Agarwal, K.,K. Singla, S., Arora, G. & Bal, C. 177Lu-EDTMP for palliation of pain from bone metastases in patients with prostate and breast cancer: a phase II study. Eur. J. Nucl. Med. Mol. Imaging 42, 79–88 (2015).
von Moos, R. et al. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Support Care Cancer 21, 3497–3507 (2013).
Gater, A. B. et al. Pain in castration-resistant prostate cancer with bone metastases: a qualitative study. Health Qual. Life Outcomes 9, 88 (2011).
Autio, K. A. et al. Prevalence of pain and analgesic use in men with metastatic prostate cancer using a patient-reported outcome measure. J. Oncol. Pract. 9, 223–229 (2013).
DeMuro, C. et al. Reasons for rejection of patient-reported outcome label claims: a compilation based on a review of patient-reported outcome use among new molecular entities and biologic license applications, 2006-2010. Value Health 15, 443–448 (2012).
Chen, R. C. et al. Recommended patient-reported core set of symptoms to measure in prostate cancer treatment trials. J. Natl Cancer Inst. 106, 1–7 (2014).
Morgans, A. K. et al. Development of a standardized set of patient-centered outcomes for advanced prostate cancer: an international effort for a unified approach. Eur. Urol. 68, 891–898 (2015).
Szende, A. Oppe, M. & Devlin, N. EQ-5D Value Sets: Inventory, Comparative Review and User Guide (Springer, 2007).
Aaronsson, N. K. et al. The European Organisation for Research and Treatment of Cancer QLQ C-30: a quality of life instrument for use in international clinical trials in oncology. J. Natl Cancer Inst. 85, 365–376 (1993).
Borghede, G. & Sullivan, M. Measurement of quality of life in localised prostatic cancer patient treated with radiotherapy: development of a prostate cancer specific module supplementing the EORTC QLQ-C30. Qual. Life Res. 5, 212–222 (1996).
Esper, P. et al. Measuring quality of life in men with prostate cancer using the Functional Assessment of Cancer Therapy-prostate instrument. Urology 50, 920–928 (1997).
Cleeland, C. S. & Ryan, K. M. Pain assessment: global use of the Brief Pain Inventory. Ann. Acad. Med. Singapore 23, 129–138 (1994).
Melzack, R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1, 277–299 (1975).
McCaffery, M. & Pasero, C. Pain: clinical manual. J. Clin. Nursing 9, 650 (2000).
Mendoza, T. R. et al. The rapid assessment of fatigue in cancer patients: use of the brief fatigue inventory. 5, 1186–1196 (1999).
Author information
Authors and Affiliations
Contributions
V.J. and L.F. researched data for this article, all authors made a substantial contribution to discussions of content, V.J. and L.F. wrote the manuscript and all authors made a substantial contribution to reviewing and/or editing the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
L.F. has acted as a consultant for and received honoraria from Amgen, Astellas and Sanofi. The other authors declare no competing interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Fallowfield, L., Payne, H. & Jenkins, V. Patient-reported outcomes in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol 13, 643–650 (2016). https://doi.org/10.1038/nrclinonc.2016.100
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.100
This article is cited by
-
Harnessing big data to characterize immune-related adverse events
Nature Reviews Clinical Oncology (2022)
-
Implementation of patient-reported outcome measures into health care for men with localized prostate cancer
Nature Reviews Urology (2022)
-
Corosolic acid, a natural triterpenoid, induces ER stress-dependent apoptosis in human castration resistant prostate cancer cells via activation of IRE-1/JNK, PERK/CHOP and TRIB3
Journal of Experimental & Clinical Cancer Research (2018)
-
Patient-reported outcomes for patients with metastatic castration-resistant prostate cancer receiving docetaxel and Atrasentan versus docetaxel and placebo in a randomized phase III clinical trial (SWOG S0421)
Journal of Patient-Reported Outcomes (2018)