Key Points
-
The discovery of androgen dependence in prostate cancer in 1941 by Huggins and colleagues still remains the backbone for the treatment of advance-staged prostate cancer
-
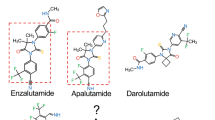
Over the past decade, the better understanding of the mechanisms driving resistance to castration has led to development of abiraterone acetate and enzalutamide, two next-generation androgen receptor (AR) targeting agents
-
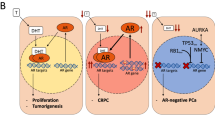
Several challenges remain including resistance to continued blocking of the AR axis, cross-resistance between agents, ideal sequence of administration, combination strategies, and coordination with other treatments
-
Molecular characterization of patients, through next-generation sequencing or transcriptome analysis, will help to dissect mechanisms of resistance and to identify biomarkers allowing the selection of patients for specific treatment
-
The improved understanding of intra-patient molecular heterogeneity and of the multiple, redundant and compensatory signalling networks in cancers supports a role for rational combinatorial, targeted therapeutic approaches
Abstract
The discovery of androgen dependence in prostate cancer in 1941 by Huggins and colleagues has remained the backbone for the treatment of this disease. However, although many patients initially respond to androgen depletion therapy, they almost invariably relapse and develop resistance with transition of the disease to a castration-resistant state. Over the past decade, the better understanding of the mechanisms that drive resistance to castration has led to the development of next-generation androgen receptor targeting agents such as abiraterone acetate and enzalutamide. This Review aims to revisit the discovery and evolution of androgen receptor targeting therapeutics for the treatment of advanced-stage prostate cancer over the years and to discuss the upcoming future and challenges in the treatment of this common cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011).
Huggins, C., Stephens, R. E. & Hodges, C. V. Studies on prostate cancer II. The effects of castration on advanced carcinoma of the prostate. Arch. Surg. 43, 209–223 (1941).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2001).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Omlin, A. et al. Improved survival in a cohort of trial participants with metastatic castration-resistant prostate cancer demonstrates the need for updated prognostic nomograms. Eur. Urol. 64, 300–306 (2013).
Walshe, W. H. The Nature and Treatment of Cancer (London: Taylor and Walton, 1846).
Lytton, B. Prostate cancer: a brief history and the discovery of hormonal ablation treatment. J. Urol. 165, 1859–1862 (2001).
Chang, C. S., Kokontis, J. & Liao. S. T. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 240, 324–326 (1988).
The Nobel Prize in Physiology or Medicine 1966. Nobelprize.org [online], (2014).
Veterans Administration Cooperative Urological Research Group. Treatment and survival of patients with cancer of the prostate. Surg. Gynecol. Obstet. 124, 1011–1017 (1967).
Schally, A. V., Kastin, A. J. & Arimura, A. Hypothalamic FSH and LH-regulating hormone: structure, physiology and clinical studies. Fertil. Steril. 22, 703–721 (1971).
Schally, A. V. et al. Peptide analogs in the therapy of prostate cancer. Prostate 45, 158–166 (2000).
Vilchez-Martinez, J. A., Pedroza, E., Arimura, A. & Schally, A. V. Paradoxical effects of D-Trp6-luteinizing hormone-releasing hormone on the hypothalamicpituitary-gonadal axis in immature female rats. Fertil. Steril. 31, 677–682 (1979).
Sandow, J., Von Rechenberg, W., Jerzabek, G. & Stoll, W. Pituitary gonadotropin inhibition by a highly active analog of luteinizing hormone-releasing hormone. Fertil. Steril. 30, 205–209 (1978).
The Nobel Prize in Physiology or Medicine 1977. Nobelprize.org [online], (2014).
Seidenfeld, J. et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann. Intern. Med. 132, 566–577 (2000).
Byar, D. P. & Corle, D. K. Hormone therapy for prostate cancer: results of the Veterans Administration Cooperative Urological Research Group studies. NCI Monogr. 7, 165–170 (1988).
The Leuprolide Study Group. Leuprolide versus diethylstilbestrol for metastatic prostate cancer. N. Engl. J. Med. 311, 1281–1286 (1984).
Van Poppel, H. & Nilsson, S. Testosterone surge: rationale for gonadotropin releasing hormone blockers? Urology 71, 1001–1006 (2008).
Princivalle, M. et al. Rapid suppression of plasma testosterone levels and tumor growth in the Dunning rat model treated with degarelix, a new gonadotropin-releasing hormone antagonist. J. Pharmacol. Exp. Ther. 320, 1113–1118 (2007).
Gittelman, M. et al. A 1-year, open label, randomized phase II dose finding study of degarelix for the treatment of prostate cancer in North America. J. Urol. 180, 1986–1992 (2008).
Klotz, L. et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel group phase III study in patients with prostate cancer. BJU Int. 102, 1531–1538 (2008).
Van Poppel, H. et al. Degarelix: a novel gonadotropin-releasing hormone (GnRH) receptor blocker—results from a 1-yr, multicentre, randomised, phase 2 dosage-finding study in the treatment of prostate cancer. Eur. Urol. 54, 805–813 (2008).
Anderson, K. M. & Liao, S. Selective retention of dihydrotestosterone by prostatic nuclei. Nature 219, 277–279 (1968).
Bruchovsky, N. & Wilson, J. D. The intranuclear binding of testosterone and 5-α-androstan-17-β-ol-3-one by rat prostate. J. Biol. Chem. 243, 5953–5960 (1968).
Mainwaring, W. I. A soluble androgen receptor in the cytoplasm of rat prostate. J. Endocrinol. 45, 531–541 (1969).
Steinberg, G. D. & Isaacs, J. T. in Cancer Chemotherapy/Frontiers in Pharmacology (eds Hickman, J. & Tritton, T.) 322–343 (Blackwell, London, 1993).
Pavone-Macaluso, M. et al. Comparison of diethylstilbestrol, cyproterone acetate and medroxyprogesterone acetate in the treatment of advanced prostatic cancer: final analysis of a randomized phase III trial of the European Organization for Research on Treatment of Cancer Urological Group. J. Urol. 136, 624–631 (1986).
Liao, S., Howell, D. K. & Chang, T. M. Action of a nonsteroidal antiandrogen, flutamide, on the receptor binding and nuclear retention of 5α-dihydrotestosterone in rat ventral prostate. Endocrinology 94, 1205–1209 (1974).
US Food and Drug Administration. Flutamide (Eulexin) Label and Approval History [online], (2014).
Labrie, F. et al. New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an antiandrogen. Clin. Invest. Med. 5, 267–275 (1982).
Lefebvre, F. A. et al. Combined long-term treatment with an LHRH agonist and a pure antiandrogen blocks androgenic influence in the rat. Prostate 3, 569–578 (1982).
Schmitt, B., Bennett, C., Seidenfeld, J., Samson, D. & Wilt, T. Maximal androgenblockade for advanced prostate cancer. Cochrane Database Systematic Reviews. Issue 2. Art. No: CD001526. http://dx.doi.10.1002/14651858.CD001526 (2000).
Akaza, H. et al. Combined androgen blockade with bicalutamide for advanced prostate cancer long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer 115, 3437–3445 (2009).
Taplin, M. E. et al. Androgen receptor mutations in androgen-independent prostate cancer: cancer and Leukemia Group B Study 9663. J. Clin. Oncol. 21, 2673–2678 (2003).
Attard, G. & de Bono, J. S. Translating scientific advancement into clinical benefit for castration-resistant prostate cancer patients. Clin. Cancer Res. 17, 3867–3875 (2011).
Alva, A. & Hussain, M. The changing natural history of metastatic prostate cancer. Cancer J. 19, 19–24 (2013).
Tannock, I. F. et al. Docetaxel plus prednisone or mitoxantrone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004).
Roy, A. K. et al. Regulation of androgen action. Vit. Horm. 55, 309–352 (1998).
Roy, A. K. et al. Androgen receptor: structural domains and functional dynamics after ligand-receptor interaction. Ann. NY Acad. Sci. 949, 44–57 (2001).
Edwards, J. & Bartlett, J. M. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 1: modifications to the androgen receptor. BJU Int. 95, 1320–1326 (2005).
Heinlein, C. A. & Chang, C. Androgen receptor in prostate cancer. Endocr. Rev. 25, 276–308 (2004).
Lu, S., Jenster, G. & Epner, D. E. Androgen induction of cyclin-dependent kinase inhibitor p21 gene: role of androgen receptor and transcription factor Sp1 complex. Mol. Endocrinol. 14, 753–760 (2000).
Xu, Y., Chen, S. Y., Ross, K. N. & Balk, S. P. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and posttranscriptional increases in cyclin D proteins. Cancer Res. 66, 7783–7792 (2006).
Wang, Q. et al. Androgen receptor regulates a distinct transcription program in androgen independent prostate cancer. Cell 138, 245–256 (2009).
Cerveira, N. et al. TMPRSS2-ERG gene fusion causing ERG overexpression precedes chromosome copy number changes in prostate carcinomas and paired HGPIN lesions. Neoplasia 8, 826–832 (2006).
Tomlins, S. A. et al. Chinnaiyan, A. M. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 66, 3396–3400 (2006).
Yu, J. et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 17, 443–454 (2010).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
King, J. C. et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat. Genet. 41, 524–526 (2009).
Carver, B. S. et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat. Genet. 41, 619–624 (2009).
Chen, Y. et al. ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nat. Med. 19, 1023–1029 (2013).
Tomlins, S. A. et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia 10, 177–188 (2008).
Ryan, C. J. & Tindall, D. J. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J. Clin. Oncol. 29, 3651–3658 (2011).
Attard, G., Richards, J. & de Bono, J. S. New strategies in metastatic prostate cancer: targeting the androgen receptor signaling pathway. Clin. Cancer Res. 17, 1649–1657 (2011).
Labrie, F. Adrenal androgens and intracrinology. Semin. Reprod. Med. 22, 299–309 (2004).
Mohler, J. L. et al. The androgen axis in recurrent prostate cancer. Clin. Cancer Res. 10, 440–448 (2004).
Titus, M. A., Schell, M. J., Lih, F. B., Tomer, K. B. & Mohler, J. L. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res. 11, 4653–4657 (2005).
Page, S. T. et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J. Clin. Endocrinol. Metab. 91, 3850–3856 (2006).
Ferraldeschi, R., Sharifi, N., Auchus, R. J. & Attard, G. Molecular pathways: Inhibiting steroid biosynthesis in prostate cancer. Clin. Cancer Res. 19, 3353–3359 (2013).
Chen, C. D. et al. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 10, 33–39 (2004).
Edwards, J., Krishna, N. S., Grigor, K. M. & Bartlett, J. M. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br. J. Cancer 89, 552–556 (2003).
Holzbeierlein, J. et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am. J. Pathol. 164, 217–227 (2004).
Yap, T. A., Zivi, A., Omlin, A. & de Bono, J. S. The changing therapeutic landscape of castration-resistant prostate cancer. Nat. Rev. Clin. Oncol. 8, 597–610 (2011).
Small, E. J. et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen- independent prostate cancer patients: a phase III trial (CALGB 9583). J. Clin. Oncol. 22, 1025–1033 (2004).
Sartor, A. O. et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group trial (SWOG 9426). Cancer 112, 2393–2400 (2008).
Small, E. J. & Srinivas, S. The anti-androgen withdrawal syndrome. Experience in a large cohort of unselected patients with advanced prostate cancer. Cancer 76, 1428–1434 (1995).
Sadar, M. D. Small molecule inhibitors targeting the “achilles' heel” of androgen receptor activity. Cancer Res. 71, 1208–1213 (2011).
Hu, R. et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 69, 16–22 (2009).
Sun, S. et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J. Clin. Invest. 120, 2715–2730 (2010).
Jenster, G. et al. Identification of two transcription activation units in the N.-terminal domain of the human androgen receptor. J. Biol. Chem. 270, 7341–7346 (1995).
Whitaker, H. C. & Neal, D. E. RAS pathways in prostate cancer—mediators of hormone resistance? Curr. Cancer Drug Targets 10, 834–839 (2010).
Wu, J. D. et al. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J. Cell Biochem. 99, 392–401 (2006).
Wen, Y. et al. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 60, 6841–6845 (2000).
Craft, N., Shostak, Y., Carey, M. & Sawyers, C. L. A mechanism for hormone independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat. Med. 5, 280–285 (1999).
Signoretti, S. et al. Her-2- neu expression and progression toward androgen independence in human prostate cancer. J. Natl Cancer Inst. 92, 1918–1925 (2000).
Yuan, X. et al. Androgen receptor remains critical for cell-cycle progression in androgen-independent CWR22 prostate cancer cells. Am. J. Pathol. 169, 682–696 (2006).
Carver, B. S. et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19, 575–586 (2011).
Chandarlapaty, S. et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19, 58–71 (2011).
Mulholland, D. J. et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 19, 792–804 (2011).
Attard, G., Belldegrun, A. S. & de Bono, J. S. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 96, 1241–1246 (2005).
Trachtenberg, J., Halpern, N. & Pont, A. Ketoconazole: a novel and rapid treatment for advanced prostatic cancer. J. Urol. 130, 152–153 (1983).
Loose, D. S., Kan, P. B., Hirst, M. A., Marcus, R. A. & Feldman, D. Ketoconazole blocks adrenal steroidogenesis by inhibiting cytochrome P450-dependent enzymes. J. Clin. Invest. 71, 1495–1499 (1983).
Potter, G. A., Barrie, S. E., Jarman, M. & Rowlands, M. G. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J. Med. Chem. 38, 2463–2471 (1995).
Jarman, M., Barrie, S. E., Leung, C. S. & Rowlands, M. G. Selective inhibition of cholesterol side-chain cleavage by potential pro-drug forms of aminoglutethimide. Anticancer Drug Des. 3, 185–190 (1988).
Attard, G. et al. Phase I clinical trial of selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 26, 4563–4571 (2008).
Danila, D. C. et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castrastion-resistant prostate cancer. J. Clin. Oncol. 28, 1496–1501 (2010).
Reid, A. H. et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J. Clin. Oncol. 28, 1489–1495 (2010).
Ryan, C. J. et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration- resistant prostate cancer who received prior ketoconazole therapy. J. Clin. Oncol. 28, 1481–1488 (2010).
Attard, G. et al. Clinical and biochemical consequences of cyp17a1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J. Clin. Endocrinol. Metab. 97, 507–516 (2012).
Fizazi, K. et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 13, 983–992 (2012).
Lorente, D. et al. Tumor responses after steroid switch of prednisolone (P) to dexamethasone (D) in castration-resistant prostate cancer (CRPC) patients (pts) on abiraterone acetate (AA) [abstract]. Eur. J. Cancer 49 (Suppl. 2), a2918 (2013).
Sharifi, N. Steroid receptors aplenty in prostate cancer. N. Engl. J. Med. 370, 970–971 (2014).
Dreicer, R. et al. Safety, pharmacokinetics, and efficacy of TAK-700 in castration-resistant, metastatic prostate cancer: A phase I/II, open-label study [abstract]. J. Clin. Oncol. 28 (Suppl. 15), a3084 (2010).
Agus, D. B. et al. Safety, efficacy, and pharmacodynamics of the investigational agent orteronel (TAK-700) in metastatic castration-resistant prostate cancer (mCRPC): updated data from a phase I/II study [abstract]. J. Clin. Oncol. 30 (Suppl. 5), a98 (2012).
George, D. J. C. P. et al. Safety and activity of the investigational agent orteronel (ortl) without prednisone in men with non-metastatic castration-resistant prostate cancer (nmCRPC) and rising prostate-specific antigen (PSA): updated results of a phase II study [abstract]. J. Clin. Oncol. 30 (Suppl.), a4549 (2012).
Petrylak, D. et al. A phase I/II study of safety and efficacy of orteronel (TAK-700), an oral, investigational, nonsteroidal 17,20-lyase inhibitor, with docetaxel and prednisone (DP) in metastatic castration-resistant prostate cancer (mCRPC): Updated phase II results [abstract]. J. Clin. Oncol. 31 (Suppl. 6), a59 (2013).
Hussain, M. et al. Safety, efficacy, and health-related quality of life (HRQoL) of the investigational single agent orteronel (ortl) in nonmetastatic castration-resistant prostate cancer (nmCRPC). J. Clin. Oncol. 31 (Suppl.), a5076 (2013).
Takeda Newsroom July—September 2013. Takeda Pharmaceutical Company Limited [online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Handratta, V. D. et al. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J. Med. Chem. 48, 2972–2984 (2005).
Vasaitis, T. et al. Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol. Cancer Ther. 7, 2348–2357 (2008).
Bruno, R. D., Gover, T. D., Burger, A. M., Brodie, A. M. & Njar, V. C. 17 alpha-Hydroxylase/17,20 lyase inhibitor VN/124–121 inhibits growth of androgen-independent prostate cancer cells via induction of the endoplasmic reticulum stress response. Mol. Cancer Ther. 7, 2828–2836 (2008).
Schayowitz, A., Sabnis, G., Njar, V. C., Brodie, A. M. Synergistic effect of a novel anti-androgen, VN/124–1, and signal transduction inhibitors in prostate cancer progression to hormone independence in vitro. Mol. Cancer Ther. 7, 121–132 (2008).
Taplin, M. E. C. F. et al. ARMOR1: safety of galeterone (TOK-001) in a phase 1 clinical trial in chemotherapy naive patients with castration resistant prostate cancer (CRPC) [abstract]. Cancer Res. 72 (Suppl. 8), CT-07 (2012).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Eisner, J. R. et al. VT-464: A novel, selective inhibitor of P450c17(CYP17)-17,20 lyase for castration-refractory prostate cancer (CRPC) [abstract]. J. Clin. Oncol. 30 (Suppl. 5), a198 (2012).
Figg, W. D. et al. Activity of oral VT-464, a selective CYP17-lyase inhibitor, in the LNCaP prostate cancer xenograft [abstract]. J. Clin. Oncol. 30 (Suppl. 5), a4671 (2012).
EU Clinical Trials Register. ClinicalTrialsRegister.eu [online], (2014).
Stanbrough, M. et al. Increased expression of genes converting adrenal androgens to testosterone in androgen independent prostate cancer. Cancer Res. 66, 2815–2825 (2006).
Fung, K. M. et al. Increased expression of type 2 3α- hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr. Relat. Cancer 13, 169–180 (2006).
Labrie, F. et al. The key role of 17 β-hydroxysteroid dehydrogenases in sex steroid biology. Steroids 62, 148–158 (1997).
Lin, H. K. et al. Expression and characterization of recombinant type 2 3α-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3α/17β-HSD activity and cellular distribution. Mol. Endocrinol. 11, 1971–1984 (1997).
Kikuchi, A. et al. ASP9521, a novel, selective, orally bioavailable AKR1C3 (type 5,17β-hydroxysteroid dehydrogenase) inhibitor: In vitro and in vivo characterization [abstract]. J. Clin. Oncol. 31 (Suppl.), a5046 (2013).
Loriot, Y. et al. Safety, tolerability and anti-tumour activity of the androgen biosynthesis inhibitor ASP9521 in patients with progressive metastatic castration-resistant prostate cancer (mCRPC): Multi-centre phase I/II study [abstract]. Eur. J. Cancer 49 (Suppl. 3), LBA22 (2013).
Montgomery, R. B. et al. Maintenance of intratumoral androgens in the metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454 (2008).
Chen, Y., Clegg, N. J. & Scher, H. I. Antiandrogens and androgen depleting therapies in prostate cancer: novel agents for an established target. Lancet Oncol. 10, 981–991 (2009).
Scher, H. I. & Sawyers, C. Biology of progressive castration resistant prostate cancer: directed therapies targeting the androgen receptor signaling axis. J. Clin. Oncol. 23, 8253–8261 (2005).
Ferraldeschi, R., Pezaro, C., Karavasilis, V. & de Bono, J. Abiraterone and novel antiandrogens: overcoming castration resistance in prostate cancer. Annu. Rev. Med. 64, 1–13 (2012).
Jung, M. E. et al. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration resistant prostate cancer. J. Med. Chem. 53, 2779–2796 (2010).
Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009).
Scher, H. I. et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 375, 1437–1446 (2010).
US Food and Drug Administration. Enzulatamide (Xtandi Capsules) [online], (2012).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Medivation. Medivation Investor Relations [online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Clegg, N. J. et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 72, 1494–1503 (2012).
Rathkopf, D. E. et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J. Clin Oncol. 31, 3525–3530 (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Rathkopf, D. E. et al. A phase II study of the androgen signaling inhibitor ARN-509 in patients with castration resistant prostate cancer (CRPC) [abstract]. J. Clin. Oncol. 30 (Suppl.), TPS4697 (2012).
Smith, M. R. et al. ARN-509 in men with high-risk non-metastatic castration-resistant prostate cancer [abstract]. Ann. Oncol. 23 (Suppl. 9), ix303, a920P (2012).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Sadar, M. D. Small molecule inhibitors targeting the ''achilles' heel'' of androgen receptor activity. Cancer Res. 71, 1208–1213 (2011).
Andersen, R. J. et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell 17, 535–546 (2010).
Loddick, S. A. et al. AZD3514: a small molecule that modulates androgen receptor signaling and function in vitro and in vivo. Mol. Cancer Ther. 9, 1715–1727 (2013).
Omlin, A. G. et al. A first-in-human study of the oral selective androgen receptor down-regulating drug (SARD) AZD3514 in patients with castration-resistant prostate cancer (CRPC) [abstract]. J. Clin. Oncol. 31 (Suppl.), a4511 (2013).
Gleave, M. E. & Monia, B. P. Antisense therapy for cancer. Nat. Rev. Cancer 5, 468–479 (2005).
Zhang, Y. et al. Reduced expression of the androgen receptor by third generation of antisense shows antitumor activity in models of prostate cancer. Mol. Cancer Ther. 10, 2309–2319 (2011).
Bianchini, D. et al. First-in-human phase I study of EZN-4176, a locked nucleic acid antisense oligonucleotide (LNA-ASO) to androgen receptor (AR) mRNA in patients with castration-resistant prostate cancer (CRPC). Br. J. Cancer 109, 2579–2586 (2013).
Massard, C. et al. ARADES trial: A first-in-man, open-label, phase I/II safety, pharmacokinetic, and proof-of-concept study of ODM-201 in patients (pts) with progressive metastatic castration-resistant prostate cancer (mCRPC) [abstract]. Ann. Oncol. 23 (Suppl. 9), ixe16, LBA25_PR (2012).
Fizazi, K. et al. An open-label, phase I/II safety, pharmacokinetic, and proof-of concept study of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (CRPC) [abstract]. Eur. J. Cancer 49 (Suppl. 2), a2853 (2013).
US National Library of Medicine. ClinicalTrials.gov[online] (2014).
US National Library of Medicine. ClinicalTrials.gov[online] (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Mezynski, J. et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann. Oncol. 11, 2943–2947 (2012).
Bianchini, D. et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur. J. Cancer 50, 78–84 (2014).
Schrader, A. J. et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur. J. Cancer 65, 30–36 (2014).
Loriot, Y. et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann. Oncol. 24, 1807–1812 (2013).
Noonan, K. L. et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann. Oncol. 24, 1802–1807 (2013).
Li, Y. et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 73, 483–489 (2013).
Carver, B. S. et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19, 575–586 (2011).
Sahu, B. et al. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 73, 1570–1580 (2013).
Richards, J. et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 72, 2176–2182 (2012).
Darshan, M. S. et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 71, 6019–6029 (2011).
Zhu, M. L. et al. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 70, 7992–8002 (2010).
Gravis, G. et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 14, 149–158 (2013).
ECOG-ACRIN Cancer Research Group Press Release. ECOG-ACRIN Cancer Research Group [online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Barbieri, C. E. et al. The mutational landscape of prostate cancer. Eur. Urol. 64, 567–576 (2013).
Lorente, D. & De Bono, J. S. Molecular alterations and emerging targets in castration resistant prostate cancer. Eur. J. Cancer 50, 753–764 (2014).
Al-Lazikani, B., Banerji, U. & Workman, P. Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 30, 679–692 (2012).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Miller, G. M. & Hinman, F. Jr. Cortisone treatment in advanced carcinoma of the prostate. J. Urol. 72, 485–496 (1954).
Author information
Authors and Affiliations
Contributions
Y.N.S.W. researched data for the article. Y.N.S.W. and R.F. made a substantial contribution to discussion of the content. Y.N.S.W., R.F. and J.d.B. wrote the article and all authors reviewed and edited the article prior to submission.
Corresponding author
Ethics declarations
Competing interests
All authors are employees of The Institute of Cancer Research, which has a commercial interest in abiraterone acetate. J.d.B. has served as a paid consultant for Astellas Pharma Inc., Janssen Biotech, Medivation and Sanofi.
Rights and permissions
About this article
Cite this article
Wong, Y., Ferraldeschi, R., Attard, G. et al. Evolution of androgen receptor targeted therapy for advanced prostate cancer. Nat Rev Clin Oncol 11, 365–376 (2014). https://doi.org/10.1038/nrclinonc.2014.72
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.72
This article is cited by
-
USP54 is a potential therapeutic target in castration-resistant prostate cancer
BMC Urology (2024)
-
Lysine methyltransferase SMYD2 enhances androgen receptor signaling to modulate CRPC cell resistance to enzalutamide
Oncogene (2024)
-
Systematic in vitro analysis of therapy resistance in glioblastoma cell lines by integration of clonogenic survival data with multi-level molecular data
Radiation Oncology (2023)
-
Targeting sex steroid biosynthesis for breast and prostate cancer therapy
Nature Reviews Cancer (2023)
-
LncFALEC recruits ART5/PARP1 and promotes castration-resistant prostate cancer through enhancing PARP1-meditated self PARylation
Cellular Oncology (2023)