Abstract
Advances in hardware and software have enabled the realization of clinically feasible, quantitative multimodality imaging of tissue pathophysiology. Earlier efforts relating to multimodality imaging of cancer have focused on the integration of anatomical and functional characteristics, such as PET–CT and single-photon emission CT (SPECT–CT), whereas more-recent advances and applications have involved the integration of multiple quantitative, functional measurements (for example, multiple PET tracers, varied MRI contrast mechanisms, and PET–MRI), thereby providing a more-comprehensive characterization of the tumour phenotype. The enormous amount of complementary quantitative data generated by such studies is beginning to offer unique insights into opportunities to optimize care for individual patients. Although important technical optimization and improved biological interpretation of multimodality imaging findings are needed, this approach can already be applied informatively in clinical trials of cancer therapeutics using existing tools. These concepts are discussed herein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pisano, E. D. et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N. Engl. J. Med. 353, 1773–1783 (2005).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Yankeelov, T. E. & Gore, J. C. Dynamic-contrast enhanced magnetic resonance imaging in oncology: theory, data acquisition, analysis, and examples. Curr. Med. Imaging Rev. 3, 91–107 (2009).
Ingrisch, M. & Sourbron, S. Tracer-kinetic modeling of dynamic contrast-enhanced MRI and CT: a primer. J. Pharmacokinet. Pharmacodyn. 40, 281–300 (2013).
Cosgrove, D. & Lassau, N. Imaging of perfusion using ultrasound. Eur. J. Nucl. Med. Mol. Imaging 37 (Suppl. 1), S65–S85 (2010).
Lewis, J. S. & Welch, M. J. PET imaging of hypoxia. Q. J. Nucl. Med. 45, 183–188 (2001).
Kurihara, H., Honda, N., Kono, Y. & Arai, Y. Radiolabelled agents for PET imaging of tumor hypoxia. Curr. Med. Chem. 19, 3282–3289 (2012).
Yankeelov, T. E., Pickens, D. R. & Price, R. R. (eds) Quantitative MRI of Cancer 193–202 (CRC Press, 2012) [Series Ed. Hendee, W. R. Imaging in Medical Diagnosis and Therapy].
He, X. & Yablonskiy, D. A. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn. Reson. Med. 57, 115–126 (2007).
Anderson, A. W. et al. Effects of cell volume fraction changes on apparent diffusion in human cells. Magn. Reson. Imaging 18, 689–695 (2000).
Tehrani, O. S. & Shields, A. F. PET imaging of proliferation with pyrimidines. J. Nucl. Med. 54, 903–912 (2013).
Czernin, J. in PET: Molecular Imaging and its Biological Implications (ed. Phelps, M. E.) 321–388 (Springer, New York, 2004).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Sundararajan, L., Linden, H. M., Link, J. M., Krohn, K. A. & Mankoff, D. A. 18F-Fluoroestradiol. Semin. Nucl. Med. 37, 470–476 (2007).
Meng, Q. & Li, Z. Molecular imaging probes for diagnosis and therapy evaluation of breast cancer. Int. J. Biomed. Imaging 2013, 230487 (2013).
Treglia, G. et al. The role of positron emission tomography using carbon-11 and fluorine-18 choline in tumors other than prostate cancer: a systematic review. Ann. Nucl. Med. 26, 451–461 (2012).
Kuhl, D. E., Hale, J. & Eaton, W. L. Transmission scanning: a useful adjunct to conventional emission scanning for accurately keying isotope deposition to radiographic anatomy. Radiology 87, 278–284 (1966).
Liew, S. C. & Hasegawa, B. H. Noise, resolution, and sensitivity considerations in the design of a single-slice emission-transmission computed tomographic system. Med. Phys. 18, 1002–1015 (1991).
Lang, T. F. et al. Description of a prototype emission-transmission computed tomography imaging system. J. Nucl. Med. 33, 1881–1887 (1992).
Kalki, K. et al. Myocardial perfusion imaging with a combined X-ray CT and SPECT system. J. Nucl. Med. 38, 1535–1540 (1997).
Beyer, T. et al. A combined PET/CT scanner for clinical oncology. J. Nucl. Med. 41, 1369–1379 (2000).
Kinahan, P. E., Townsend, D. W., Beyer, T. & Sashin, D. Attenuation correction for a combined 3D PET/CT scanner. Med. Phys. 25, 2046–2053 (1998).
Poeppel, T. D., Krause, B. J., Heusner, T. A., Boy, C., Bockisch, A. & Antoch, G. PET/CT for the staging and follow-up of patients with malignancies. Eur. J. Radiol. 70, 382–392 (2009).
Antoch, G. et al. Non-small cell lung cancer: dual-modality PET/CT in preoperative staging. Radiology 229, 526–533 (2003).
Lardinois, D. et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N. Engl. J. Med. 348, 2500–2507 (2003).
Cohade, C., Osman, M., Leal, J. & Wahl, R. L. Direct comparison of 18F-FDG PET and PET/CT in patients with colorectal carcinoma. J. Nucl. Med. 44, 1797–1803 (2003).
Schöder, H., Yeung, H. W., Gonen, M., Kraus, D. & Larson, S. M. Head and neck cancer: clinical usefulness and accuracy of PET/CT image fusion. Radiology 231, 65–72 (2004).
Pfannenberg, A. C. et al. Benefit of anatomical-functional image fusion in the diagnostic work-up of neuroendocrine neoplasms. Eur. J. Nucl. Med. Mol. Imaging 30, 835–843 (2003).
Even-Sapir, E., Keidar, Z. & Bar-Shalom, R. Hybrid imaging (SPECT/CT and PET/CT)--improving the diagnostic accuracy of functional/metabolic and anatomic imaging. Semin. Nucl. Med. 39, 264–275 (2009).
Sauter, A. W., Wehrl, H. F., Kolb, A., Judenhofer, M. S. & Pichler, B. J. Combined PET/MRI: one step further in multimodality imaging. Trends Mol. Med. 16, 508–515 (2010).
Judenhofer, M. S. et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat. Med. 14, 459–465 (2008).
Yankeelov, T. E. et al. Simultaneous PET-MRI in oncology: a solution looking for a problem? Magn. Reson. Imaging 30, 1342–1356 (2012).
Buchbender, C., Heusner, T. A., Lauenstein, T. C., Bockisch, A. & Antoch, G. Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis. J. Nucl. Med. 53, 928–938 (2012).
Huang, S. H. et al. A comparative study of fused FDG PET/MRI, PET/CT, MRI, and CT imaging for assessing surrounding tissue invasion of advanced buccal squamous cell carcinoma. Clin. Nucl. Med. 36, 518–525 (2011).
Tatsumi, M. et al. 18F-FDG PET/MRI fusion in characterizing pancreatic tumors: comparison to PET/CT. Int. J. Clin. Oncol. 16, 408–415 (2011).
Catalano, O. A. et al. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients—a hypothesis-generating exploratory study. Radiology 269, 857–869 (2013).
Borgwardt, L. et al. Increased fluorine-18 2-fluoro-2-deoxy-D-glucose (FDG) uptake in childhood CNS tumors is correlated with malignancy grade: a study with FDG positron emission tomography/magnetic resonance imaging coregistration and image fusion. J. Clin. Oncol. 23, 3030–3037 (2005).
Nagamachi, S. et al. The usefulness of 18F-FDG PET/MRI fusion image in diagnosing pancreatic tumor: comparison with 18F-FDG PET/CT. Ann. Nucl. Med. 27, 554–563 (2013).
Pfluger, T. et al. Diagnostic value of combined 18F-FDG PET/MRI for staging and restaging in paediatric oncology. Eur. J. Nucl. Med. Mol. Imaging 39, 1745–1755 (2012).
Walter, C. et al. Clinical and diagnostic value of preoperative MR mammography and FDG-PET in suspicious breast lesions. Eur. Radiol. 13, 1651–1656 (2003).
Zonari, P., Baraldi, P. & Crisi, G. Multimodal MRI in the characterization of glial neoplasms: the combined role of single-voxel MR spectroscopy, diffusion imaging and echo-planar perfusion imaging. Neuroradiology 49, 795–803 (2007).
Yabuuchi, H. et al. Parotid gland tumors: can addition of diffusion-weighted MR imaging to dynamic contrast-enhanced MR imaging improve diagnostic accuracy in characterization? Radiology 249, 909–916 (2008).
Mazaheri, Y. et al. Prostate cancer: identification with combined diffusion-weighted MR imaging and 3D 1H MR spectroscopic imaging—correlation with pathologic findings. Radiology 246, 480–484 (2008).
Segal, E. et al. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat. Biotechnol. 25, 675–680 (2007).
Gevaert, O. et al. Non-small cell lung cancer: identifying prognostic imaging biomarkers by leveraging public gene expression microarray data--methods and preliminary results. Radiology 264, 387–396 (2012).
Gutman, D. A. et al. MR imaging predictors of molecular profile and survival: multi-institutional study of the TCGA glioblastoma data set. 267, 560–569 (2013).
Zinn, P. O. et al. Radiogenomic mapping of edema/cellular invasion MRI-phenotypes in glioblastoma multiforme. PLoS ONE 6, e25451 (2011).
Choi, H. Response evaluation of gastrointestinal stromal tumors. Oncologist 13 (Suppl. 2), 4–7 (2008).
Yanagawa, M. et al. Evaluation of response to neoadjuvant chemotherapy for esophageal cancer: PET response criteria in solid tumors versus response evaluation criteria in solid tumors. J. Nucl. Med. 53, 872–880 (2012).
Teng, F. F., Meng, X., Sun, X. D. & Yu, J. M. New strategy for monitoring targeted therapy: molecular imaging. Int. J. Nanomedicine 8, 3703–3713 (2013).
Jansen, J. F. et al. Tumor metabolism and perfusion in head and neck squamous cell carcinoma: pretreatment multimodality imaging with 1H magnetic resonance spectroscopy, dynamic contrast-enhanced MRI, and [18F]FDG-PET. Int. J. Radiat. Oncol. Biol. Phys. 82, 299–307 (2012).
Thorwarth, D., Eschmann, S. M., Holzner, F., Paulsen, F. & Alber, M. Combined uptake of [18F]FDG and [18F]FMISO correlates with radiation therapy outcome in head-and-neck cancer patients. Radiother. Oncol. 80, 151–156 (2006).
Ling, C. C. et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int. J. Radiat. Oncol. Biol. Phys. 47, 551–560 (2000).
Vera, P. et al. Simultaneous positron emission tomography (PET) assessment of metabolism with 18F-fluoro-2-deoxy-d-glucose (FDG), proliferation with 18F-fluoro-thymidine (FLT), and hypoxia with 18Fluoro-misonidazole (F-MISO) before and during radiotherapy in patients with non-small-cell lung cancer (NSCLC): a pilot study. Radiother. Oncol. 98, 109–116 (2011).
Giganti, F. et al. Response to chemotherapy in gastric adenocarcinoma with diffusion-weighted MRI and 18F-FDG-PET/CT: Correlation of apparent diffusion coefficient and partial volume corrected standardized uptake value with histological tumor regression grade. J. Magn. Reson. Imaging http://dx.doi.org/10.1002/jmri.24464.
Galldiks, N. et al. Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J. Nucl. Med. 53, 1048–1057 (2012).
Buchbender, C., Heusner, T. A., Lauenstein, T. C., Bockisch, A. & Antoch, G. Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis. J. Nucl. Med. 53, 928–938 (2012).
Buchbender, C., Heusner, T. A., Lauenstein, T. C., Bockisch, A. & Antoch, G. Oncologic PET/MRI, part 2: bone tumors, soft-tissue tumors, melanoma, and lymphoma. J. Nucl. Med. 53, 1244–1252 (2012).
Rajendran, J. G. et al. [18F]FMISO and [18F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur. J. Nucl. Med. Mol. Imaging 30, 695–704 (2003).
Cherk, M. H. et al. Lack of correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in non–small cell lung cancer assessed by 18F-fluoromisonidazole and 18F-FDG PET. J. Nucl. Med. 47, 1921–1926 (2006).
Komar, G. et al. Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin. Cancer Res. 15, 5511–5517 (2009).
Kamel, I. R. et al. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology 250, 466–473 (2009).
Jenkinson, M. D. et al. Phase II trial of intratumoral BCNU injection and radiotherapy on untreated adult malignant glioma. J. Neurooncol. 99, 103–113 (2010).
Clarke, L. P. et al. The Quantitative Imaging Network: NCI's historical perspective and planned goals. Transl. Oncol. 7, 1–4 (2014).
QIBA Protocols and Profiles. Radiological Society of North America [online], (2014).
ECOG-ACRIN cancer research group. ECOG-AGRIN.org [online], (2014).
Yankeelov, T. E. et al. Clinically relevant modeling of tumor growth and treatment response. Sci. Transl. Med. 5, 187ps9 (2013).
Szeto, M. D. et al. Quantitative metrics of net proliferation and invasion link biological aggressiveness assessed by MRI with hypoxia assessed by FMISO-PET in newly diagnosed glioblastomas. Cancer Res. 69, 4502–4509 (2009).
Atuegwu, N. C. et al. Parameterizing the logistic model of tumor growth by DW-MRI and DCE-MRI data to predict treatment response and changes in breast cancer cellularity during neoadjuvant chemotherapy. Transl. Oncol. 6, 256–264 (2013).
Hogea, C., Davatzikos, C. & Biros, G. An image-driven parameter estimation problem for a reaction–diffusion glioma growth model with mass effects. J. Math. Biol. 56, 793–825 (2008).
Yankeelov, T. E., Arlinghaus, L. R., Li, X. & Gore, J. C. The role of magnetic resonance imaging biomarkers in clinical trials of treatment response in cancer. Semin. Oncol. 38, 16–25 (2011).
Thakur, M. L. & Lentle, B. C. Report of a summit on molecular imaging. J. Nucl. Med. 46, 11N–42N (2005).
Jain, R. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Batchelor, T. T. et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11, 83–95 (2007).
Sorensen, A. G. et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 69, 5296–5300 (2009).
Sorensen, A. G. et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 72, 402–407 (2012).
Emblem, K. E. et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat. Med. 19, 1178–1183 (2013).
Ribatti, D. Vascular normalization: a real benefit? Cancer Chemother. Pharmacol. 68, 275–278 (2011).
Vangestel, C. et al. 99mTc-(CO)3 His-annexin A5 micro-SPECT demonstrates increased cell death by irinotecan during the vascular normalization window caused by bevacizumab. J. Nucl. Med. 52, 1786–1794 (2011).
Vangestel, C. et al. 99mTc-(CO)3 His-annexin A5 micro-SPECT demonstrates increased cell death by irinotecan during the vascular normalization window caused by bevacizumab.. J. Nucl. Med. 52, 1786–1794 (2011).
Acknowledgements
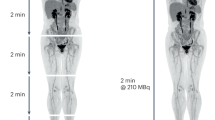
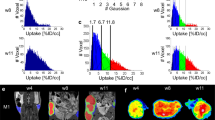
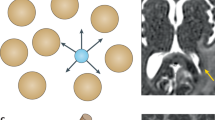
T.E.Y. thanks the National Cancer Institute for funding support through grants 1U01CA142565, 1U01CA174706, R01 CA138599, R25CA092043. R.G.A. is funded in part by the AUR GE Radiology Research Academic Fellowship. C.C.Q. thanks the Vanderbilt Ingram Cancer Center Young Ambassadors grant and NCI 1R01CA158079. The authors thank the Kleberg Foundation for generously supporting their imaging programme and NCI P30CA68485 (PI: J. Pietenpol). The authors also thank Drs J. Skinner and X. Li of the Vanderbilt University Institute of Imaging Science, Nashville, TN, USA, for preparing Figure 2 and Figure 3, respectively.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to all stages of the preparation of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
T.E.Y. has been a consultant for Eli Lilly. R.G.A. and C.C.Q. declare no competing interests.
Rights and permissions
About this article
Cite this article
Yankeelov, T., Abramson, R. & Quarles, C. Quantitative multimodality imaging in cancer research and therapy. Nat Rev Clin Oncol 11, 670–680 (2014). https://doi.org/10.1038/nrclinonc.2014.134
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.134
This article is cited by
-
Multimodal prediction of neoadjuvant treatment outcome by serial FDG PET and MRI in women with locally advanced breast cancer
Breast Cancer Research (2023)
-
Mathematical modelling of the dynamics of image-informed tumor habitats in a murine model of glioma
Scientific Reports (2023)
-
Omniparticle Contrast Agent for Multimodal Imaging: Synthesis and Characterization in an Animal Model
Molecular Imaging and Biology (2023)
-
Spatially resolved isotope tracing reveals tissue metabolic activity
Nature Methods (2022)
-
Criteria for the design of tissue-mimicking phantoms for the standardization of biophotonic instrumentation
Nature Biomedical Engineering (2022)