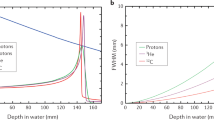
Abstract
The use of charged particle therapy to control tumours non-invasively offers advantages over conventional radiotherapy. Protons and heavy ions deposit energy far more selectively than X-rays, allowing a higher local control of the tumour, a lower probability of damage to healthy tissue, low risk of complications and the chance for a rapid recovery after therapy. Charged particles are also useful for treating tumours located in areas that surround tissues that are radiosensitive and in anatomical sites where surgical access is limited. Current trial outcomes indicate that accelerated ions can potentially replace surgery for radical cancer treatments, which might be beneficial as the success of surgical cancer treatments are largely dependent on the expertise and experience of the surgeon and the location of the tumour. However, to date, only a small number of controlled randomized clinical trials have made comparisons between particle therapy and X-rays. Therefore, although the potential advantages are clear and supported by data, the cost:benefit ratio remains controversial. Research in medical physics and radiobiology is focusing on reducing the costs and increasing the benefits of this treatment.
Key Points
-
Charged particle therapy (CPT) is an emerging technique in radiotherapy, with several new centres under construction worldwide, despite their high cost compared to conventional X-ray therapy
-
Protons are ideal for conformal treatment, and are useful for treating paediatric tumours; reduced late morbidity is expected because of the lower integral dose to the normal tissue
-
Heavy ions (carbon) provide physical and biological advantages compared with X-rays, such as high relative biological effectiveness and reduced oxygen enhancement ratio in the tumour region
-
Clinical trials are still too scarce to draw firm conclusions on the cost effectiveness of CPT
-
Spot-scanning provides the best dose profiles compared with passive beam modulation, but requires corrections for treating moving targets
-
Research in radiobiology and combined therapies is necessary to define the optimum tumours to be treated with CPT
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Thariat, J., Hannoun-Levi, J. M., Sun Myint, A., Vuong, T. & Gérard, J. P. Past, present, and future of radiotherapy for the benefit of patients. Nat. Rev. Clin. Oncol. 10, 52–60 (2013).
Jaffray, D. A. Image-guided radiotherapy: from current concept to future perspectives. Nat. Rev. Clin. Oncol. 9, 688–699 (2012).
Bortfeld, T. IMRT: a review and preview. Phys. Med. Biol. 51, R363–R379 (2006).
Lo, S. S. et al. Stereotactic body radiation therapy: a novel treatment modality. Nat. Rev. Clin. Oncol. 7, 44–54 (2010).
Kavanagh, B. D., Timmerman, R. & Meyer, J. L. The expanding roles of stereotactic body radiation therapy and oligofractionation: toward a new practice of radiotherapy. Front. Radiat. Ther. Oncol. 43, 370–381 (2011).
Durante, M. & Loeffler, J. S. Charged particles in radiation oncology. Nat. Rev. Clin. Oncol. 7, 37–43 (2010).
Schardt, D., Elsässer, T. & Schulz-Ertner, D. Heavy-ion tumor therapy: physical and radiobiological benefits. Rev. Mod. Phys. 82, 383–425 (2010).
Grün, R. et al. Impact of enhancements in the local effect model (LEM) on the predicted RBE-weighted target dose distribution in carbon ion therapy. Phys. Med. Biol. 57, 7261–7274 (2012).
Particle Therapy Co-operative Group. PTCOG Home [online], (2013).
The Advisory Board Company. Technology Insights. Proton Beam Therapy [online], (2011).
De Ruysscher, D. et al. Charged particles in radiotherapy: a 5-year update of a systematic review. Radiother. Oncol. 103, 5–7 (2012).
Durante, M. Eighth Warren K. Sinclair keynote address: Heavy ions in therapy and space: benefits and risks. Health. Phys. 103, 532–539 (2012).
Castro, J. R. Results of heavy ion radiotherapy. Radiat. Environ. Biophys. 34, 45–48 (1995).
DOE-NCI. Workshop on Ion Beam Therapy—Summary Report. January 9–11 [online], (2013).
Allen, A. M. et al. An evidence based review of proton beam therapy: the report of ASTRO's emerging technology committee. Radiother. Oncol. 103, 8–11 (2012).
Suit, H. et al. Should positive phase III clinical trial data be required before proton beam therapy is more widely adopted? No. Radiother. Oncol. 86, 148–153 (2008).
Sheets, N. C. et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 307, 1611–1620 (2012).
Vargas, C. et al. Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 70, 744–751 (2008).
Mendenhall, N. P., Schild, S. & Slater, J. Radiation therapy modalities for prostate cancer. JAMA 308, 450–451 (2012).
Deville, C., Ben-Josef, E. & Vapiwala, N. Radiation therapy modalities for prostate cancer. JAMA 308, 451 (2012).
Penson, D. F. Re: Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. J. Urol. 188, 2230–2231 (2012).
Roelofs, E. et al. Results of a multicentric in silico clinical trial (ROCOCO): comparing radiotherapy with photons and protons for non-small cell lung cancer. J. Thorac. Oncol. 7, 165–176 (2012).
Brada, M., Pijls-Johannesma, M. & De Ruysscher, D. Proton therapy in clinical practice: current clinical evidence. J. Clin. Oncol. 25, 965–970 (2007).
Schulz-Ertner, D. & Tsujii, H. Particle radiation therapy using proton and heavier ion beams. J. Clin. Oncol. 25, 953–964 (2007).
Brada, M., Pijls-Johannesma, M. & De Ruysscher, D. Current clinical evidence for proton therapy. Cancer J. 15, 319–324 (2009).
Tsujii, H. et al. Clinical advantages of carbon ion radiotherapy. New J. Phys. 10, 075009 (2008).
Terasawa, T. et al. Systematic review: charged-particle radiation therapy for cancer. Ann. Intern. Med. 151, 556–565 (2009).
Tsujii, H. & Kamada, T. A review of update clinical results of carbon ion radiotherapy. Jpn J. Clin. Oncol. 42, 670–685 (2012).
Ogino, T. Clinical evidence of particle beam therapy (proton). Int. J. Clin. Oncol. 17, 79–84 (2012).
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 63, 11–30 (2013).
Newhauser, W. D. & Durante, M. Assessing the risk of second malignancies after modern radiotherapy. Nat. Rev. Cancer 11, 438–448 (2011).
Kuhlthau, K. A. et al. Prospective study of health-related quality of life for children with brain tumors treated with proton radiotherapy. J. Clin. Oncol. 30, 2079–2086 (2012).
Hoppe, B. S. et al. Improving the therapeutic ratio in Hodgkin lymphoma through the use of proton therapy. Oncology (Williston Park) 26, 456–459 (2012).
Hodgson, D. C. Late effects in the era of modern therapy for Hodgkin lymphoma. Hematology Am. Soc. Hematol. Educ. Program 2011, 323–329 (2011).
Li, J. et al. Rationale for and preliminary results of proton beam therapy for mediastinal lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 81, 167–174 (2011).
Hoppe, B. S. et al. Effective dose reduction to cardiac structures using protons compared with 3DCRT and IMRT in mediastinal Hodgkin lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 82, 449–455 (2012).
Combs, S. E. et al. Carbon ion radiotherapy for pediatric patients and young adults treated for tumors of the skull base. Cancer 115, 1348–1355 (2009).
Combs, S. E. et al. Treatment of pediatric patients and young adults with particle therapy at the Heidelberg Ion Therapy Center (HIT): establishment of workflow and initial clinical data. Radiat. Oncol. 7, 170 (2012).
Yock, T. I. & Caruso, P. A. Risk of second cancers after photon and proton radiotherapy: a review of the data. Health Phys. 103, 577–585 (2012).
Zhang, R. et al. Comparison of risk of radiogenic second cancer following photon and proton craniospinal irradiation for a pediatric medulloblastoma patient. Phys. Med. Biol. 58, 807–823 (2013).
Paganetti, H. Assessment of the risk for developing a second malignancy from scattered and secondary radiation in radiation therapy. Health Phys. 103, 652–661 (2012).
Winkfield, K. M. et al. Modeling intracranial second tumor risk and estimates of clinical toxicity with various radiation therapy techniques for patients with pituitary adenoma. Technol. Cancer Res. Treat. 10, 243–251 (2011).
Brenner, D. J. et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc. Natl Acad. Sci. USA 100, 13761–13766 (2003).
Mullenders, L., Atkinson, M., Paretzke, H., Sabatier, L. & Bouffler, S. Assessing cancer risk of low-dose radiation. Nat. Rev. Cancer 9, 596–604 (2009).
Pearce, M. S. et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380, 499–505 (2012).
Brenner, D. J. & Hall, E. J. Computed tomography—an increasing source of radiation exposure. N. Engl. J. Med. 357, 2277–2284 (2007).
Hall, E. J. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 65, 1–7 (2006).
Kaderka, R. et al. Out-of-field dose measurements in a water phantom using different radiotherapy modalities. Phys. Med. Biol. 57, 5059–5074 (2012).
La Tessa, C. et al. Out-of-field dose studies with an anthropomorphic phantom: comparison of X-rays and particle therapy treatments. Radiother. Oncol. 105, 133–138 (2012).
Münter, M. W. et al. Heavy ion radiotherapy during pregnancy. Fertil. Steril. 94, 2329.e5–7 (2010).
Halperin, E. C. Particle therapy and treatment of cancer. Lancet Oncol. 7, 676–685 (2006).
Toyama, S. et al. Long-term results of carbon ion radiation therapy for locally advanced or unfavorably located choroidal melanoma: usefulness of CT-based 2-port orthogonal therapy for reducing the incidence of neovascular glaucoma. Int. J. Radiat. Oncol. Biol. Phys. 86, 270–276 (2013).
McGovern, S. L. & Mahajan, A. Progress in radiotherapy for pediatric sarcomas. Curr. Oncol. Rep. 14, 320–326 (2012).
Barker, J. L. Jr, Paulino, A. C., Feeney, S., McCulloch, T. & Hoffman, H. Locoregional treatment for adult soft tissue sarcomas of the head and neck: an institutional review. Cancer J. 9, 49–57 (2003).
Ciernik, I. F. et al. Proton-based radiotherapy for unresectable or incompletely resected osteosarcoma. Cancer 117, 4522–4530 (2011).
Laramore, G. E. The use of neutrons in cancer therapy: a historical perspective through the modern era. Semin. Oncol. 24, 672–685 (1997).
Matsunobu, A. et al. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer 118, 4555–4463 (2012).
Imai, R. et al. Effect of carbon ion radiotherapy for sacral chordoma: results of phase I–II and phase II clinical trials. Int. J. Radiat. Oncol. Biol. Phys. 77, 1470–1476 (2010).
Nishida, Y. et al. Clinical outcome of sacral chordoma with carbon ion radiotherapy compared with surgery. Int. J. Radiat. Oncol. Biol. Phys. 79, 110–116 (2011).
Schulz-Ertner, D. et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int. J. Radiat. Oncol. Biol. Phys. 68, 449–457 (2007).
Nikoghosyan, A. V. et al. Randomised trial of proton vs. carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-study. BMC Cancer 10, 607 (2010).
Nikoghosyan, A. V. et al. Randomised trial of proton vs. carbon ion radiation therapy in patients with low and intermediate grade chondrosarcoma of the skull base, clinical phase III study. BMC Cancer 10, 606 (2010).
Simone, C. B. 2nd et al. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother. Oncol. 101, 376–382 (2011).
van der Water, T. A., Bijl, H. P., Schilstra, C., Pijls-Johannesma, M. & Langendijk, J. A. The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature. Oncologist 16, 366–377 (2011).
Ramaekers, B. L. et al. Systematic review and meta-analysis of radiotherapy in various head and neck cancers: comparing photons, carbon-ions and protons. Cancer Treat. Rev. 37, 185–201 (2011).
Schulz-Ertner, D. et al. Therapy strategies for locally advanced adenoid cystic carcinomas using modern radiation therapy techniques. Cancer 104, 338–344 (2005).
Tanaka, S., Louis, D. N., Curry, W. T., Batchelor, T. T. & Dietrich, J. Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nat. Rev. Clin. Oncol. 10, 14–26 (2013).
Yang, I. & Aghi, M. K. New advances that enable identification of glioblastoma recurrence. Nat. Rev. Clin. Oncol. 6, 648–657 (2009).
Mizumoto, M. et al. Phase I/II trial of hyperfractionated concomitant boost proton radiotherapy for supratentorial glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 77, 98–105 (2010).
Combs, S. E. et al. Randomized phase II study evaluating a carbon ion boost applied after combined radiochemotherapy with temozolomide versus a proton boost after radiochemotherapy with temozolomide in patients with primary glioblastoma: the CLEOPATRA trial. BMC Cancer 10, 478 (2010).
Chen, J. et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488, 522–526 (2012).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006).
Evers, P. et al. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer 10, 384 (2010).
Lee, P. et al. Evaluation of high ipsilateral subventricular zone radiation therapy dose in glioblastoma: a pooled analysis. Int. J. Radiat. Oncol. Biol. Phys. http://dx.doi.org/10.1016/j.ijrobp.2013.01.009.
Gupta, T. et al. Can irradiation of potential cancer stem-cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma? J. Neurooncol. 109, 195–203 (2012).
Blechacz, B. & Mishra, L. Hepatocellular carcinoma biology. Recent Results Cancer Res. 190, 1–20 (2013).
El-Seragh, H. B. Hepatocellular carcinoma. N. Eng. J. Med. 365, 1118–1127 (2011).
Bush, D. A., Hillebrand, D. J., Slater, J. M. & Slater, J. D. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology 127 (Suppl. 1), S189–S193 (2004).
Fukumitsu, N. et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 74, 831–836 (2009).
Imada, H. et al. Comparison of efficacy and toxicity of short-course carbon ion radiotherapy for hepatocellular carcinoma depending on their proximity to the porta hepatis. Radiother. Oncol. 96, 231–235 (2010).
Combs, S. E. et al. Phase I study evaluating the treatment of patients with hepatocellular carcinoma (HCC) with carbon ion radiotherapy: the PROMETHEUS-01 trial. BMC Cancer 11, 67 (2011).
Stathis, A. & Moore, M. J. Advanced pancreatic carcinoma: current treatment and future challenges. Nat. Rev. Clin. Oncol. 7, 163–172 (2010).
Terashima, K. et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother. Oncol. 103, 25–31 (2012).
Shinoto, M. et al. Phase 1 trial of preoperative, short-course carbon-ion radiotherapy for patients with resectable pancreatic cancer. Cancer 119, 45–51 (2013).
Nielsen, M. B., Rasmussen, P. C., Lindegaard, J. C. & Laurberg, S. A 10-year experience of total pelvic exenteration for primary advanced and locally recurrent rectal cancer based on a prospective database. Colorectal Dis. 14, 1076–1083 (2012).
Mobaraki, A., Ohno, T., Yamada, S., Sakurai, H. & Nakano, T. Cost-effectiveness of carbon ion radiation therapy for locally recurrent rectal cancer. Cancer Sci. 101, 1834–1839 (2010).
Combs, S. E. et al. Phase I/II trial evaluating carbon ion radiotherapy for the treatment of recurrent rectal cancer: the PANDORA-01 trial. BMC Cancer 12, 137 (2012).
Pécuchet, N., Fournie, L. S. & Oudard, S. New insights into the management of renal cell cancer. Oncology 84, 22–31 (2013).
Nomiya, T. et al. Carbon ion radiation therapy for primary renal cell carcinoma: initial clinical experience. Int. J. Radiat. Oncol. Biol. Phys. 72, 828–833 (2008).
Sisterson, J. Ion beam therapy in 2004. Nucl. Instr. Meth. B 241, 713–716 (2005).
Yu, J. B. et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J. Natl Cancer Inst. 105, 25–32 (2013).
Zietman, A. L. et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 294, 1233–1239 (2005).
Talcott, J. A. et al. Patient-reported long-term outcomes after conventional and high-dose combined proton and photon radiation for early prostate cancer. JAMA 303, 1046–1053 (2010).
Zietman, A. L. et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95–09. J. Clin. Oncol. 28, 1106–1111 (2010).
Hoppe, B. S. et al. Proton therapy for prostate cancer. Oncology (Williston Park) 25, 644–650 (2011).
Henderson, R. H. et al. Urinary functional outcomes and toxicity five years after proton therapy for low- and intermediate-risk prostate cancer: results of two prospective trials. Acta Oncol. 52, 463–469 (2013).
Gray, P. J. & Efstathiou, J. A. Proton beam radiation therapy for prostate cancer-is the hype (and the cost) justified? Curr. Urol. Rep. http://dx.doi.org/10.1007/s11934-013-0320–2.
Vogelius, I. R. & Bentzen, S. M. Meta-analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: bad news, good news, or no news? Int. J. Radiat. Oncol. Biol. Phys. 85, 89–94 (2013).
Brenner, D. J. Toward optimal external-beam fractionation for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 48, 315–316 (2000).
Kil, W. J. et al. Hypofractionated passively scattered proton radiotherapy for low- and intermediate-risk prostate cancer is not associated with post-treatment testosterone suppression. Acta Oncol. 52, 492–497 (2013).
Ishikawa, H. et al. Carbon-ion radiation therapy for prostate cancer. Int. J. Urol. 19, 296–305 (2012).
Zhang, X. et al. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer: a virtual clinical study. Int. J. Radiat. Oncol. Biol. Phys. 77, 357–366 (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Fridberg, S. & Rudén, B. I. Hypofractionation in radiotherapy. An investigation of injured Swedish women, treated for cancer of the breast. Acta Oncol. 48, 822–831 (2009).
Freedman, G. M. Hypofractionated radiation therapy in the treatment of early-stage breast cancer. Curr. Oncol. Rep. 14, 12–19 (2012).
Appelt, A. L., Vogelius, I. R. & Bentzen, S. M. Modern hypofractionation schedules for tangential whole breast irradiation decrease the fraction size-corrected dose to the heart. Clin. Oncol. (R. Coll. Radiol.) 25, 147–152 (2013).
Bush, D. A. et al. Partial breast irradiation delivered with proton beam: results of a phase II trial. Clin. Breast Cancer 11, 241–245 (2011).
Bert, C., Engenhart-Cabillic, R. & Durante, M. Particle therapy for noncancer diseases. Med. Phys. 39, 1716–1727 (2012).
Kjellberg, R. N., Hanamura, T., Davis, K. R., Lyons, S. L. & Adams, R. D. Bragg-peak proton-beam therapy for arteriovenous malformations of the brain. N. Engl. J. Med. 309, 269–274 (1983).
Barker, F. G. 2nd et al. Dose-volume prediction of radiation-related complications after proton beam radiosurgery for cerebral arteriovenous malformations. J. Neurosurg. 99, 254–263 (2003).
Chen, C. C., Chapman, P., Petit, J. & Loeffler, J. Proton radiosurgery in neurosurgery. Neurosurg. Focus 23, E5 (2007).
Halasz, L. M. et al. Proton stereotactic radiosurgery for the treatment of benign meningiomas. Int. J. Radiat. Oncol. Biol. Phys. 81, 1428–1435 (2011).
Loeffler, J. S. & Shih, H. A. Radiation therapy in the management of pituitary adenomas. J. Clin. Endocrinol. Metab. 96, 1992–2003 (2011).
Lazzara, B. M. et al. Cyberknife radiosurgery in treating trigeminal neuralgia. J. Neurointerv. Surg. 5, 81–85 (2013).
Kishan, A. U., Modjtahedi, B. S., Morse, L. S. & Lee, P. Radiation therapy for neovascular age-related macular degeneration. Int. J. Radiat. Oncol. Biol. Phys. 85, 583–597 (2013).
Terasawa, T. et al. Systematic review: comparative effectiveness of radiofrequency catheter ablation for atrial fibrillation. Ann. Intern. Med. 151, 191–202 (2009).
Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376, 1903–1909 (2010).
Freeman, T. Will protons gradually replace photons? Medical Physics Web [online], (2012).
Korreman, S. et al. The European Society of Therapeutic Radiology and Oncology-European Institute of Radiotherapy (ESTRO-EIR) report on 3D CT-based in-room image guidance systems: a practical and technical review and guide. Radiother. Oncol. 94, 129–144 (2010).
Korreman, S. S. Motion in radiotherapy: photon therapy. Phys. Med. Biol. 57, R161–R191 (2012).
Bert, C. & Durante, M. Motion in radiotherapy: particle therapy. Phys. Med. Biol. 56, R113–R144 (2011).
Silari, M. Applications of particle accelerators in medicine. Radiat. Prot. Dosim. 146, 440–450 (2011).
Bartal, T. et al. Focusing of short-pulse high-intensity laser-accelerated proton beams. Nat. Phys. 8, 139–142 (2012).
Robin, D. S. et al. Superconducting toroidal combined-function magnet for a compact ion beam cancer therapy gantry. Nucl. Instrum. Meth. 659, 484–493 (2011).
Miyamoto, T. et al. Curative treatment of Stage I non-small cell lung cancer with carbon ion beams using a hypofractionated regimen. Int. J. Radiat. Oncol. Biol. Phys. 67, 750–758 (2007).
Graeff, C., Durante, M. & Bert, C. Motion mitigation in intensity modulated particle therapy by internal target volumes covering range changes. Med. Phys. 39, 6004–6013 (2012).
Knopf, A. et al. Special report: workshop on 4D-treatment planning in actively scanned particle therapy—recommendations, technical challenges, and future research directions. Med. Phys. 37, 4608–4614 (2010).
Riboldi, M., Orecchia, R. & Baroni, G. Real-time tumour tracking in particle therapy: technological developments and future perspectives. Lancet Oncol. 13, e383–e391 (2012).
Mumot, M. et al. Proton range verification using a range probe: definition of concept and initial analysis. Phys. Med. Biol. 55, 4771–4782 (2010).
Durante, M. & Stöcker, H. Relativistic protons for image-guided stereotactic radiosurgery. J. Phys. Conf. Ser. 373, 012016 (2012).
Friedrich, T., Scholz, U., Elsässer, T., Durante, M. & Scholz, M. Systematic analysis of RBE and related quantities using a database of cell survival experiments with ion beam irradiation. J. Radiat. Res. http://dx.doi.org/10.1093/jrr/rrs114.
Paganetti, H. et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 53, 407–421 (2002).
Frese, M. C. et al. Application of constant vs. variable relative biological effectiveness in treatment planning of intensity-modulated proton therapy. Int. J. Radiat. Oncol. Biol. Phys. 79, 80–88 (2011).
Li, Q. & Sihver, L. Therapeutic techniques applied in the heavy-ion therapy at IMP. Nucl. Instr. Meth. B 269, 664–670 (2011).
Baumann, M., Krause, M. & Hill, R. Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer 8, 545–554 (2008).
Keith, B. & Simon, M. C. Hypoxia-inducible factors, stem cells, and cancer. Cell 129, 465–472 (2007).
Pignalosa, D. & Durante, M. Overcoming resistance of cancer stem cells. Lancet Oncol. 13, e187–e188 (2012).
Malanchi, I. et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 (2011).
Cui, X. et al. Effects of carbon ion beam on putative colon cancer stem cells and its comparison with X-rays. Cancer Res. 71, 3676–3687 (2011).
Oonishi, K. et al. Different effects of carbon ion beams and X-rays on clonogenic survival and DNA repair in human pancreatic cancer stem-like cells. Radiother. Oncol. 105, 258–265 (2012).
Quan, Y. et al. Accumulation efficiency of cancer stem-like cells post γ-ray and proton irradiation. Nucl. Instr. Meth. B 286, 341–345 (2012).
Fu, Q. et al. Response of cancer stem-like cells and non-stem cancer cells to proton and γ-ray irradiation. Nucl. Instr. Meth. B 286, 346–350 (2012).
Garcia-Barros, M. et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300, 1155–1159 (2003).
Deng, X. et al. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science 322, 110–115 (2008).
Yamada, Y. et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int. J. Radiat. Oncol. Biol. Phys. 71, 484–490 (2008).
Takahashi, Y. et al. Heavy ion irradiation inhibits in vitro angiogenesis even at sublethal dose. Cancer Res. 63, 4253–4257 (2003).
Combs, S. E. et al. In vitro evaluation of photon and carbon ion radiotherapy in combination with chemotherapy in glioblastoma cells. Radiat. Oncol. 7, 9 (2012).
Combs, S. E. et al. Radiobiological evaluation and correlation with the local effect model (LEM) of carbon ion radiation therapy and temozolomide in glioblastoma cell lines. Int. J. Radiat. Biol. 85, 126–137 (2009).
Kirkwood, J. M. et al. Immunotherapy of cancer in 2012. CA Cancer J. Clin. 62, 309–335 (2012).
Kaminski, J. M. The controversial abscopal effect. Cancer Treat. Rev. 31, 159–172 (2005).
Postow, M. A. et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 366, 925–931 (2012).
Hiniker, S. M. et al. Abscopal effect in a patient with melanoma. N. Engl. J. Med. 366, 2035 (2012).
Shiraishi, K. et al. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1 alpha. Clin. Cancer Res. 14, 1159–1166 (2008).
Formenti, S. C. & Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 10, 718–726 (2009).
Formenti, S. C. & Demaria, S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J. Natl Cancer Inst. 105, 256–265 (2013).
Ogata, T. et al. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res. 65, 113–120 (2005).
Ogata, T. et al. Carbon ion irradiation suppresses metastatic potential of human non-small cell lung cancer A549 cells through the phosphatidylinositol-3-kinase/Akt signaling pathway. J. Radiat. Res. 52, 374–379 (2011).
Matsunaga, A. et al. Carbon-ion beam treatment induces systemic antitumor immunity against murine squamous cell carcinoma. Cancer 116, 3740–3748 (2010).
Horsman, M. R., Mortensen, L. S., Petersen, J. B., Busk, M. & Overgaard, J. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 9, 674–687 (2012).
Ogawa, K. et al. Radiotherapy targeting cancer stem cells: current views and future perspectives. Anticancer Res. 33, 747–754 (2013).
Zelefsky, M. J. et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 82, 1744–1788 (2012).
GSI Helmholtzzentrum für Schwerionenforschung. PIDE Project [online], (2013).
Acknowledgements
We thank Michael Scholz, Christoph Bert, Christian Graeff, and Thomas Friedrich for providing some of the figures.
Author information
Authors and Affiliations
Contributions
M. Durante researched the data for the article and wrote the article. Both authors made a substantial contribution to discussion of content and J. S. Loeffler reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J. S. Loeffler declares he is on the Scientific Advisory Board of Procure. M. Durante declares no competing interests.
Rights and permissions
About this article
Cite this article
Loeffler, J., Durante, M. Charged particle therapy—optimization, challenges and future directions. Nat Rev Clin Oncol 10, 411–424 (2013). https://doi.org/10.1038/nrclinonc.2013.79
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2013.79
This article is cited by
-
High efficacy of particle beam therapies against tumors under hypoxia and prediction of the early stage treatment effect using 3'-deoxy-3'-[18F]fluorothymidine positron emission tomography
Annals of Nuclear Medicine (2024)
-
Proton beam therapy for the isolated recurrence of endometrial cancer in para-aortic lymph nodes: a case report
BMC Women's Health (2022)
-
Thermal neutron as a potential mutagen for induced plant mutation breeding: radiosensitivity response on wheat and rice
Genetic Resources and Crop Evolution (2022)
-
Smart material based on boron crosslinked polymers with potential applications in cancer radiation therapy
Scientific Reports (2021)
-
Inter-patient variations in relative biological effectiveness for cranio-spinal irradiation with protons
Scientific Reports (2020)