Abstract
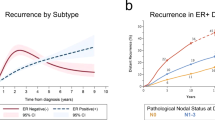
The reactivation of cancer cells following a seemingly successful treatment of the primary tumour with initial therapies (such as tumour excision or systemic therapy) is a well-known phenomenon. This metastatic rebirth is preceded by an interlude, termed dormancy, when cancer sleeps undetected for periods that can last years or even decades. Discoveries over the past 10 years have revealed the therapeutic potential of prolonging dormancy for maintaining a clinically asymptomatic state, or the permanent clearance of dormant residual disseminated cancer cells to affect a 'cure'. Here, we provide an overview of the mechanisms of dormancy and use genitourinary cancers as models to demonstrate how dormancy principles could be exploited clinically. Data from these models have yielded promising therapeutic strategies to address dormancy as well as diagnostics that could enable clinicians to monitor the dormant state of cancer in patients. This Review also aims to convey that dormancy, as a whole, likely results from coalescing contributions made by each of the three types of dormancy discussed (cellular, angiogenic and immunological). In our opinion, dormancy-directed therapies will prove most effective when the effect of these cumulative contributions are understood and targeted.
Key Points
-
Although the concept of cancer dormancy has been considered for over 120 years, it has been largely overlooked as a regulator of disease progression until the past two decades
-
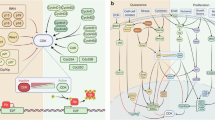
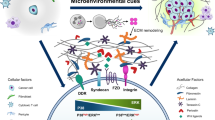
Cancer dormancy can be currently explained by three mechanisms (cellular, angiogenic and immunological), with each making important contributions to the dormant state as a whole
-
Preclinical studies are providing an understanding of dormancy leading to therapeutic strategies to maintain or induce dormancy, or alternatively clear residual dormant cells from patients in remission to affect a permanent cure
-
New immunotherapies and antiangiogenic agents have been approved in the past decade and these may provide new tools to test the proposed mechanisms of dormancy in the clinical setting
-
Technological advances are emerging that might enable safe, effective and low-cost, long-term monitoring of dormancy required for clinical assessment of dormancy, and the evaluation of therapeutic efficacy in future clinical trials
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Talmadge, J. E., Wolman, S. R. & Fidler, I. J. Evidence for the clonal origin of spontaneous metastases. Science 217, 361–363 (1982).
Willis, R. A. The Spread of Tumours in the Human Body (J. & A. Churchill, London, 1934).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1, 571–573 (1889).
Hadfield, G. The dormant cancer cell. Br. Med. J. 4888, 607–610 (1954).
Norton, L. A Gompertzian model of human breast cancer growth. Cancer Res. 48, 7067–7071 (1988).
Demicheli, R. et al. Local recurrences following mastectomy: support for the concept of tumor dormancy. J. Natl Cancer Inst. 86, 45–48 (1994).
Demicheli, R., Abbattista, A., Miceli, R., Valagussa, P. & Bonadonna, G. Time distribution of the recurrence risk for breast cancer patients undergoing mastectomy: further support about the concept of tumor dormancy. Breast Cancer Res. Treat. 41, 177–185 (1996).
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971).
Quesnel, B. Tumor dormancy and immunoescape. APMIS 116, 685–694 (2008).
Muller, V. et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin. Cancer Res. 11, 3678–3685 (2005).
Aguirre-Ghiso, J. A. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 7, 834–846 (2007).
Pantel, K., Brakenhoff, R. H. & Brandt, B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat. Rev. Cancer 8, 329–340 (2008).
Hou, J. M. et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 5, 525–532 (2012).
Rameshwar, P. Breast cancer cell dormancy in bone marrow: potential therapeutic targets within the marrow microenvironment. Expert Rev. Anticancer Ther. 10, 129–132 (2010).
Meng, S. et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 10, 8152–8162 (2004).
Luzzi, K. J. et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 153, 865–873 (1998).
Cameron, D. M. et al. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 60, 2541–2546 (2000).
Lim, P. K. et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 71, 1550–1560 (2011).
Kiel, M. J. & Morrison, S. J. Uncertainty in the niches that maintain haematopoietic stem cells. Nat. Rev. Immunol. 8, 290–301 (2008).
Lu, X. et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 20, 701–714 (2011).
Gewirtz, D. A. Autophagy, senescence and tumor dormancy in cancer therapy. Autophagy 5, 1232–1234 (2009).
Wang, Q. et al. Survivin and escaping in therapy-induced cellular senescence. Int. J. Cancer 128, 1546–1558 (2011).
Kobayashi, A. et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in the bone. J. Exp. Med. 208, 2641–2655 (2011).
Mazar, A. P., Ahn, R. W. & O'Halloran, T. V. Development of novel therapeutics targeting the urokinase plasminogen activator receptor (uPAR) and their translation toward the clinic. Curr. Pharm. Des. 17, 1970–1978 (2011).
Aguirre-Ghiso, J. A., Kovalski, K. & Ossowski, L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell Biol. 147, 89–103 (1999).
Aguirre-Ghiso, J. A., Ossowski, L. & Rosenbaum, S. K. Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 64, 7336–7345 (2004).
Aguirre-Ghiso, J. A., Liu, D., Mignatti, A., Kovalski, K. & Ossowski, L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol. Biol. Cell 12, 863–879 (2001).
Aguirre-Ghiso, J. A., Estrada, Y., Liu, D. & Ossowski, L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res. 63, 1684–1695 (2003).
Yu, W., Kim, J. & Ossowski, L. Reduction in surface urokinase receptor forces malignant cells into a protracted state of dormancy. J. Cell Biol. 137, 767–777 (1997).
Wang, F. et al. Reciprocal interactions between b1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc. Natl Acad. Sci. USA 95, 14821–14826 (1998).
Weaver, V. M. et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137, 231–245 (1997).
Hapke, S. et al. Integrin αvβ3/vitronectin interaction affects expression of the urokinase system in human ovarian cancer cells. J. Biol. Chem. 28, 26340–26348 (2001).
Hickson, J. A. et al. The p38 kinases MKK4 and MKK6 suppress metastatic colonization in human ovarian carcinoma. Cancer Res. 66, 2264–2270 (2006).
Lotan, T. et al. c-Jun NH2-terminal kinase activating kinase 1/mitogen-activated protein kinase kinase 4-mediated inhibition of SKOV3ip.1 ovarian cancer metastasis involves growth arrest and p21 up-regulation. Cancer Res. 68, 2166–2175 (2008).
Connell, J. L. et al. Probing prokaryotic social behaviors with bacterial ''lobster traps''. MBio 1, e00202–e00210 (2010).
Agur, Z. et al. Disruption of a quorum sensing mechanism triggers tumorigenesis: a simple discrete model corroborated by experiments in mammary cancer stem cells. Biol. Direct 5, 20 (2010).
Hickson, J. et al. Societal interactions in ovarian cancer metastasis: a quorum-sensing hypothesis. Clin. Exp. Metastasis 26, 67–76 (2009).
Barkin, D. et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 68, 6241–6250 (2008).
Steeg, P. S. & Theodorescu, D. Metastasis: a therapeutic target for cancer. Nat. Clin. Pract. Oncol. 5, 206–219 (2008).
Holmgren, L., O'Reilly, M. S. & Folkman, J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med. 1, 149–153 (1995).
Torres Filho, I. P., Leunig, M., Yuan, F., Intaglietta, M. & Jain, R. K. Non-invasive measurement of microvascular and interstitial oxygen profiles in a human tumor in SCID mice. Proc. Natl Acad. Sci. USA 91, 2081–2085 (1994).
Naumov, G. N., Akslen, L. A. & Folkman, J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle 5, 1779–1787 (2006).
Hanahan, D. & Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86, 353–364 (1996).
Okamoto, R. et al. Hematopoietic cells regulate the angiogenic switch during tumorigenesis. Blood 105, 2757–2763 (2005).
Carmeliet, P. et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis Nature 394, 485–490 (1998).
Hicklin, D. J. & Ellis, L. M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 23, 1011–1027 (2005).
Zanetti, J. S. et al. The role of tumor hypoxia in MUC1-positive breast carcinomas. Virchows Arch. 459, 367–375 (2011).
Jouanneau, J., Moens, G., Montesano, R. & Thiery, J. P. FGF-1 but not FGF-4 secreted by carcinoma cells promotes in vitro and in vivo angiogenesis and rapid tumor proliferation. Growth Factors 12, 37–47 (1995).
Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 4, 540–550 (2004).
Bergers, G. & Coussens, L. M. Extrinsic regulators of epithelial tumor progression: metalloproteinases. Curr. Opin. Genet. Dev. 10, 120–127 (2000).
Heissig, B., Hattori, K., Friedrich, M., Rafii, S. & Werb, Z. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr. Opin. Hematol. 10, 136–141 (2003).
Rundhaug, J. E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 9, 267–285 (2005).
Folkman, J., Watson, K., Ingber, D. & Hanahan, D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 339, 58–61 (1989).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732 (2003).
Naumov, G. N. et al. A model of human tumor dormancy: an angiogenic switch from the non angiogenic phenotype. J. Natl Cancer Inst. 98, 316–325 (2006).
Giuriato, S. et al. Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch. Proc. Natl Acad. Sci. USA 103, 16266–16271 (2006).
Indraccolo, S. et al. Interruption of tumor dormancy by a transient angiogenic burst within the tumor microenvironment. Proc. Natl Acad. Sci. USA 103, 4216–4221 (2006).
Straume, O. et al. Suppression of heat shock protein 27 induces long-term dormancy in human breast cancer. Proc. Natl Acad. Sci. USA 109, 8699–8704 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Ebbinghaus, S. et al. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clin. Cancer Res. 15, 6689–6695 (2007).
Almog, N. et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 69, 836–844 (2009).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360 (2004).
Ge, W. et al. B7-H1 up-regulation on dendritic-like leukemia cells suppresses T cell immune function through modulation of IL-10/IL-12 production and generation of Treg cells. Leuk. Res. 33, 948–957 (2009).
Teng, M. W. L., Swann, J. B., Koebel, C. M., Schreiber, R. D. & Smyth, M. J. Immune-mediated dormancy: an equilibrium with cancer. J. Leukocyte Bio. 84, 988–993 (2008).
Cekic, C. et al. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J. Immunol. 188, 198–205 (2012).
Kauffman, M. H. et al. Transplant tumor registry: donor related malignancies. Transplantation 74, 358–362 (2002).
Strauss, D. C. & Thomas, J. M. Transmission of donor melanoma by organ transplantation. Lancet Oncol. 11, 790–796 (2010).
Uhr, J. W. & Pantel, K. Controversies in clinical cancer dormancy. Proc. Natl Acad. Sci. USA 108, 12396–12400 (2011).
Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 6, 295–307 (2006).
Ribatti, D. & Crivellato, E. Immune cells and angiogenesis. J. Cell. Mol. Med. 13, 2822–2833 (2009).
Rogers, T. L. & Holen, I. Tumour macrophages as potential targets of bisphosphonates. J. Transl. Med. 9, 177 (2011).
Said, N., Smith, S., Sanchez-Carbayo, M. & Theodorescu, D. Tumor endothelin-1 enhances metastatic colonization of the lung in mouse xenograft models of bladder cancer. J. Clin. Invest. 121, 132–147 (2011).
Lepisto, A. J., McKolanis, J. R. & Finn, O. J. in Cancer immunotherapy: immune suppression and tumor growth Ch. 10 (eds Prendergast, G. C. & Jaffee, E. M.) 167–176 (Academic Press, London, 2007).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Titus, B. et al. Endothelin axis is a target of the lung metastasis suppressor gene RhoGDI2. Cancer Res. 65, 7320–7327 (2005).
Said, N., Sanchez-Carbayo, M., Smith, S. C. & Theodorescu, D. RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J. Clin. Invest. 122, 1503–1518 (2012).
Steeg, P. S., Ouatas, T., Halverson, D., Palmieri, D. & Salerno, M. Metastasis suppressor genes: basic biology and potential clinical use. Clin. Breast Cancer 4, 51–62 (2003).
Nash, K. T. et al. Requirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy. J. Natl Cancer Inst. 99, 309–321 (2007).
Horak, C. E., Lee, J. H., Marshall, J. C., Shreeve, S. M. & Steeg, P. S. The role of metastasis suppressor genes in metastatic dormancy. APMIS 116, 586–601 (2008).
FDA. NME Drug and New Biologic Approvals in 2005 [online], (2011).
Motzer, R. J. et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 356, 115–124 (2007).
Posadas, E. M. & Figlin, R. A. Systemic therapy in renal cell carcinoma: advancing paradigms. Oncology 26, 290–301 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
National Cancer Institute. FDA Approval for Sipuleucel-T [online], (2010).
Small, E. J. et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 24, 3089–3094 (2006).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Glode, L. M., Barqawi, A., Crighton, F., Crawford, E. D. & Kerbel, R. Metronomic therapy with cyclophosphamide and dexamethasone for prostate carcinoma. Cancer 98, 1643–1648 (2003).
Hanahan, D., Bergers, G. & Bergsland, E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J. Clin. Invest. 105, 1045–1047 (2000).
Martin-Padura, I. et al. Residual dormant cancer stem-cell foci are responsible for tumor relapse after antiangiogenic metronomic therapy in hepatocellular carcinoma xenografts. Lab. Invest. 92, 952–966 (2012).
Pietras, K. & Hanahan, D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J. Clin. Oncol. 23, 939–952 (2005).
Bocci, G., Francia, G., Man, S., Lawler, J. & Kerbel, R. S. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc. Natl Acad. Sci. USA 100, 12917–12922 (2003).
Bocci, G., Nicolaou, K. C. & Kerbel, R. S. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 62, 6938–6943 (2002).
Loeffler, M., Kruger, J. A. & Reisfeld, R. A. Immunostimulatory effects of low-dose cyclophosphamide are controlled by inducible nitric oxide synthase. Cancer Res. 65, 5027–5030 (2005).
Ghiringhelli, F. et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 56, 641–648 (2007).
Fontana, A. et al. Metronomic cyclophosphamide in elderly patients with advanced, castration-resistant prostate cancer. J. Am. Geriatr. Soc. 58, 986–988 (2010).
Pasquier, E., Kavallaris, M. & Andre, N. Metronomic chemotherapy: new rationale for new directions. Nat. Rev. Clin. Oncol. 7, 455–465 (2010).
Flanigan, R. C. et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N. Engl. J. Med. 345, 1655–1659 (2001).
Mickisch, G. H., Garin, A., van Poppel, H., de Prijck, L. & Sylvester, R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group. Lancet 358, 966–970 (2001).
Elhilali, M. M. et al. Placebo-associated remissions in a multicentre, randomized, double-blind trial of interferon gamma-1b for the treatment of metastatic renal cell carcinoma. The Canadian Urologic Oncology Group. BJU Int. 86, 613–618 (2000).
Gunduz, N., Fisher, B. & Saffer, E. A. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res. 39, 3861–3865 (1979).
Guba, M. et al. A primary tumor promotes dormancy of solitary tumor cells before inhibiting angiogenesis. Cancer Res. 61, 5575–5579 (2001).
Smith, S. C. et al. A 20-gene model for molecular nodal staging of bladder cancer: development and prospective assessment. Lancet Oncol. 12, 137–143 (2011).
Curtis, C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012).
Brenner, D. J. & Hall, E. J. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 357, 2277–2284 (2007).
Racila, E. et al. Detection and characterization of carcinoma cells in the blood. Proc. Natl Acad. Sci. USA 95, 4589–4594 (1999).
Riethdorf, S. et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin. Cancer Res. 13, 920–928 (2007).
Sieuwerts, A. M. et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumour cells. J. Natl Cancer Inst. 101, 61–66 (2009).
van Houten, V. M. et al. Molecular assays for the diagnosis of minimal residual head-and-neck cancer: methods, reliability, pitfalls, and solutions. Clin. Cancer Res. 6, 3803–3816 (2000).
Shaw, J. A. et al. Genomic analysis of circulating cell-free DNA infers breast cancer dormancy. Genome Res. 22, 220–231 (2012).
Mitchell, P. S. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl Acad. Sci. USA 105, 10513–10518 (2008).
Marasa, B. S. et al. Increased MKK4 abundance with replicative senescence is linked to the joint reduction of multiple microRNAs. Sci. Signal. 2, ra69 (2009).
Heyn, C. et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn. Reson. Med. 56, 1001–1010 (2006).
Townson, J. L. et al. Three-dimensional imaging and quantification of both solitary cells and metastases in whole mouse liver by magnetic resonance imaging. Cancer Res. 69, 8326–8331 (2009).
Acknowledgements
This work is supported by National Institutes of Health grants CA075115 and CA104106 to DT. The authors would like to thank Dan Welch for his critical review of the manuscript and contribution of unpublished data.
Author information
Authors and Affiliations
Contributions
J. A. Hensel and T. W. Flaig researched data for the article, substantially contributed to the discussion of content and wrote the article. D. Theodorescu reviewed and edited the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hensel, J., Flaig, T. & Theodorescu, D. Clinical opportunities and challenges in targeting tumour dormancy. Nat Rev Clin Oncol 10, 41–51 (2013). https://doi.org/10.1038/nrclinonc.2012.207
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2012.207
This article is cited by
-
Long non-coding RNA NR2F1-AS1 induces breast cancer lung metastatic dormancy by regulating NR2F1 and ΔNp63
Nature Communications (2021)
-
Synergistic therapeutic effect of combined PDGFR and SGK1 inhibition in metastasis-initiating cells of breast cancer
Cell Death & Differentiation (2020)
-
NR2F1 contributes to cancer cell dormancy, invasion and metastasis of salivary adenoid cystic carcinoma by activating CXCL12/CXCR4 pathway
BMC Cancer (2019)
-
Targeting metastasis
Nature Reviews Cancer (2016)
-
Increasing radiation dose improves immunotherapy outcome and prolongation of tumor dormancy in a subgroup of mice treated for advanced intracerebral melanoma
Cancer Immunology, Immunotherapy (2016)