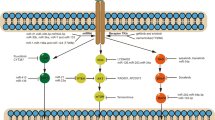
Abstract
Lung cancer is a complex and often fatal disease. The recent discovery of activating mutations in EGFR and fusion genes involving ALK has set the stage for personalized medicine for lung cancer. Patients selected using biomarkers have benefited from the development of EGFR tyrosine kinase inhibitors and ALK inhibitors with considerable improvement in tumor control and survival. Four key areas of knowledge that are essential to the development of targeted therapy are discussed in this Review: knowing the target, knowing the biomarker, knowing the end point and knowing the mechanisms of resistance.
Key Points
-
Personalized medicine should be based on a molecular target that drives cancer cell proliferation, the inhibition of which would cease tumor growth
-
Predictive biomarkers are crucial to identify patients with molecular targets, who are likely to respond better to targeted therapies than to standard therapy
-
Progression-free survival could be an appropriate primary end point in randomized comparative studies with considerable crossover, as overall survival of the two groups would be similar
-
It is likely that patients who benefit from molecular-targeted therapy will eventually become resistant to the drug; understanding the mechanisms of resistance is essential to prevent or defer this process
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J. Clin. Oncol. 26, 4617–4625 (2008).
Azzoli, C. G. et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J. Clin. Oncol. 27, 6251–6266 (2009).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Mok, T. S. et al. A double-blind placebo-controlled randomized study of Chinese herbal medicine as complementary therapy for reduction of chemotherapy-induced toxicity. Ann. Oncol. 18, 768–774 (2007).
Lu, A. P., Jia, H. W., Xiao, C. & Lu, Q. P. Theory of traditional Chinese medicine and therapeutic method of diseases. World J. Gastroenterol. 10, 1854–1856 (2004).
Ding, L. et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 455, 1069–1075 (2008).
Mok, T., Wu, Y. L. & Zhang, L. A small step towards personalized medicine for non-small cell lung cancer. Discov. Med. 8, 227–231 (2009).
Sun, S., Schiller, J. H. & Gazdar, A. F. Lung cancer in never smokers —a different disease. Nat. Rev. Cancer 7, 778–790 (2007).
Sequist, L. V., Bell, D. W., Lynch, T. J. & Haber, D. A. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J. Clin. Oncol. 25, 587–595 (2007).
Kancha, R. K., von Bubnoff, N., Peschel, C. & Duyster, J. Functional analysis of epidermal growth factor receptor (EGFR) mutations and potential implications for EGFR targeted therapy. Clin. Cancer Res. 15, 460–467 (2009).
Inoue, A. et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J. Clin. Oncol. 27, 1394–1400 (2009).
Rosell, R. et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 361, 958–967 (2009).
Tamura, K. et al. Multicentre prospective phase II trial of gefitinib for advanced non-small cell lung cancer with epidermal growth factor receptor mutations: results of the West Japan Thoracic Oncology Group trial (WJTOG0403). Br. J. Cancer 98, 907–914 (2008).
Sutani, A. et al. Gefitinib for non-small-cell lung cancer patients with epidermal growth factor receptor gene mutations screened by peptide nucleic acid-locked nucleic acid PCR clamp. Br. J. Cancer 95, 1483–1489 (2006).
Shigematsu, H. et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl Cancer Inst. 97, 339–346 (2005).
Yatabe, Y. & Mitsudomi, T. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 98, 1817–1824 (2007).
Fukuoka, M. et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 21, 2237–2246 (2003).
Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009).
Lee, J. S. et al. A randomized phase III study of gefitinib versus standard chemotherapy (gemcitabine plus cisplatin) as a first-line treatment for never-smokers with advanced or metastatic adenocarcinoma of the lung [abstract]. 13th World Conference on Lung Cancer PRS.4 (2009).
Mitsudomi, T. et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 11, 121–128 (2010).
Maemondo, M. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362, 2380–2388 (2010).
Zhou, C. et al. Efficacy results from the randomised phase III OPTIMAL (CTONG 0802) study comparing first-line erlotinib versus carboplatin (CBDCA) plus gemcitabine (GEM), in Chinese advanced non-small-cell lung cancer (NSCLC) patients with EGFR activating mutations [abstract LBA13]. Ann. Oncol. 21 (Suppl. 8), viii6 (2010).
Rosell, R. et al. Erlotinib versus chemotherapy in advanced non-small-cell lung cancer patients with epidermal growth factor receptor mutations: Interim results of the European Erlotinib vs chemotherapy (EURTAC) phase III randomized trial [abstract]. J. Clin. Oncol. 29 (Suppl.), a7503 (2011).
Shiota, M. & Mori, S. The clinicopathological features of anaplastic large cell lymphomas expressing p80NPM/ALK. Leuk. Lymphoma 23, 25–32 (1996).
Soda, M. et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448, 561–567 (2007).
Wong, D. W. et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 15, 1723–1733 (2009).
Perner, S. et al. EML4-ALK fusion lung cancer: a rare acquired event. Neoplasia 10, 298–302 (2008).
Zhang, X. et al. Fusion of EML4 and ALK is associated with development of lung adenocarcinoma lacking EGFR and KRAS mutation and is correlated with ALK expression. Mol. Cancer 9, 188 (2010).
Shaw, A. T. et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J. Clin. Oncol. 27, 4247–4253 (2009).
Kwak, E. L. et al. Anaplastic lymphoma kinase inhibition in non-small cell lung cancer. N. Engl. J. Med. 363, 1693–1703 (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 (2001).
Thatcher, N. et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small cell lung cancer: Results from a randomized, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366, 1527–1537 (2005).
Shepherd, F. A. et al. Erlotinib in previously treated non-small cell lung cancer. N. Engl. J. Med. 353, 123–132 (2005).
Kim, E. S. et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer: a randomised phase III trial (INTEREST). Lancet 372, 1809–1818 (2008).
Fukuoka, M. et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. doi:10.1200/JCO.2010.33.4235.
Kosaka, T., Yatabe, Y., Onozato, R., Kuwano, H. & Mitsudomi, T. Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J. Thorac. Oncol. 4, 22–29 (2009).
Goss, G. D. et al. A phase III prospective, randomized, double-blind, placebo-controlled trial of the epidermal growth factor receptor inhibitor gefitinib in completely resected stage IB-IIIA non-small-cell lung cancer (NSCLC): NCIC CTG BR.19 [abstract]. J. Clin. Oncol 28 (Suppl. 18), LBA7005 (2010).
Schiller, J. H. et al. Comparison of four chemotherapy regimens for advanced non-small cell lung cancer. N. Engl. J. Med. 346, 92–98 (2002).
Shepherd, F. A. et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J. Clin. Oncol. 18, 2095–2103 (2000).
Hanna, N. et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J. Clin. Oncol. 22, 1589–1597 (2004).
Riely, G. J., Marks, J. & Pao, W. KRAS mutations in non-small cell lung cancer. Proc. Am. Thorac. Soc. 6, 201–205 (2009).
Kobayashi, S. et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 352, 786–792 (2005).
Pao, W. et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2, e73 (2005).
Engelman, J. A. et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316, 1039–1043 (2007).
Bean, J. et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl Acad. Sci. USA 104, 20932–20937 (2007).
Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377 (2008).
Rosell, R. et al. Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutation. Clin. Cancer Res. 17, 1160–1169 (2011).
Oxnard, G. R. et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR mutant lung cancer: distinct natural history of patients with tumor harboring the T790M mutation. Clin. Cancer Res. 17, 1616–1622 (2011).
Belani, C. The role of irreversible EGFR inhibitors in the treatment of non-small cell lung cancer: overcoming resistance to reversible EGFR inhibitors. Cancer Invest. 28, 413–423 (2010).
Miller, V. A. et al. Phase IIb/III trial of afatinib (BIBW 2992) + best supportive care (BSC) vs. placebo + BSC in patients failing 1–2 lines of chemotherapy and erlotinib/gefitinib (LUX-Lung 1) [abstract LBA1]. Ann. Oncol. 21 (Suppl. 8), viii1 (2010).
Schiller, J. et al. Results from ARQ 197-209: a global randomized placebo-controlled phase II clinical trial of erlotinib plus ARQ 197 versus erlotinib plus placebo in previously treated EGFR inhibitor-naive patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) [abstract]. J. Clin. Oncol. 28 (Suppl. 18), a7502 (2010).
Choi, Y. L. et al. EML4–ALK mutations in lung cancer that confer resistance to ALK inhibitors. N. Engl. J. Med. 363, 1734–1739 (2010).
Jackman, K. et al. A clinical definition of acquired resistance to EGFR tyrosine kinase inhibitors in non-small-cell lung cancer. J. Clin. Oncol. 28, 357–360 (2009).
Mok, T. Living with imperfection. J. Clin. Oncol. 28, 191–192 (2009).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 92, 205–216 (2000).
Riely, G. et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin. Cancer Res. 13, 5150–5155 (2007).
Yung, T. K. et al. Single-molecule detection of epidermal growth factor receptor mutations in plasma by microfluidics digital PRC in non-small cell lung cancer patients. Clin. Cancer Res. 15, 2076–2084 (2009).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
T. S. K. Mok declares he is a consultant and receives honoraria from AstraZeneca, Boehringer Ingelheim, Merck Serono, Pfizer and Roche. He also receives research grants from AstraZeneca.
Rights and permissions
About this article
Cite this article
Mok, T. Personalized medicine in lung cancer: what we need to know. Nat Rev Clin Oncol 8, 661–668 (2011). https://doi.org/10.1038/nrclinonc.2011.126
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2011.126
This article is cited by
-
Non-Small Cell Lung Cancer Testing on Reference Specimens: An Italian Multicenter Experience
Oncology and Therapy (2024)
-
Machine learning based personalized drug response prediction for lung cancer patients
Scientific Reports (2022)
-
Longevity leap: mind the healthspan gap
npj Regenerative Medicine (2021)
-
Genetic variants in histone modification regions are associated with the prognosis of lung adenocarcinoma
Scientific Reports (2021)
-
Comprehensive genomic profile of Chinese lung cancer patients and mutation characteristics of individuals resistant to icotinib/gefitinib
Scientific Reports (2020)