Abstract
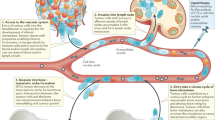
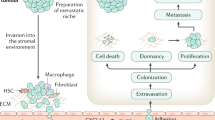
Bone is the most common metastatic site for breast cancer, and bone metastases can cause pain as well as risk of pathological fractures. Emerging treatments for metastatic bone disease have arisen from advances in our understanding of the unique cellular and molecular mechanisms that contribute to bone metastasis. The interaction between tumor cells and the bone microenvironment results in a 'vicious cycle' that increases both bone destruction and tumor burden. The tumor secretes factors, such as parathyroid hormone-related peptide, that stimulate osteoclastogenesis. Similarly, the bone stroma produces growth factors, such as transforming growth factor β, that promote tumor growth in bone. Therapeutic targeting of these microenvironmental factors is under intensive investigation. Other attractive therapeutic targets include signaling molecules, such as receptor activator of nuclear factor κB ligand, Src kinase, and cathepsin K, all of which regulate osteoclast function, and chemokine receptor 4, which is involved in the homing of tumor cells to bone. In this Review, we describe the progress and future directions of novel bone-targeted therapies that may reduce or prevent destructive bone metastasis from breast cancer. Novel modalities for predicting and monitoring treatment response will also be described.
Key Points
-
A more thorough understanding of the interaction between tumor cells and the bone microenvironment will direct the development of novel bone-targeted treatments
-
Adjuvant bisphosphonate treatments, especially zoledronic acid, may have antitumor effects that both prevent and treat bone metastasis, as well as improving survival
-
Denosumab is a promising agent that might be more effective than bisphosphonates in preventing skeletal-related events in metastatic breast cancer; whether denosumab has antitumor effects is not yet clear
-
In addition to denosumab, Src kinase inhibitors are promising agents under development for the treatment of bone metastases from breast cancer
-
Systemic radionuclide therapy may have antitumor effects that lead to improved survival
-
Imaging modalities, gene-expression signatures, bone markers, disseminated tumor cells, and circulating tumor cells are under investigation as means of directing personalized treatment
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Coleman, R. E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 27, 165–176 (2001).
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002).
Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 8, 98–101 (1989).
Roodman, G. D. Mechanisms of bone metastasis. N. Engl. J. Med. 350, 1655–1664 (2004).
Mundy, G. R. & Yoneda, T. Bisphosphonates as anticancer drugs. N. Engl. J. Med. 339, 398–400 (1998).
Clézardin, P., Fournier, P., Boissier, S. & Peyruchaud, O. In vitro and in vivo antitumor effects of bisphosphonates. Curr. Med. Chem. 10, 173–180 (2003).
Fromigue, O., Lagneaux, L. & Body, J. J. Bisphosphonates induce breast cancer cell death in vitro. J. Bone Miner. Res. 15, 2211–2221 (2000).
Ottewell, P. D. et al. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J. Natl Cancer Inst. 100, 1167–1178 (2008).
Wood, J. et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J. Pharmacol. Exp. Ther. 302, 1055–1061 (2002).
Pavlakis, N., Schmidt, R. & Stockler, M. Bisphosphonates for breast cancer. Cochrane Database of Systematic Reviews, Issue 3. Art. no.: CD003474. doi:10.1002/14651858.CD003474.pub2 (2005).
Kristensen, B. et al. Bisphosphonate treatment in primary breast cancer: results from a randomised comparison of oral pamidronate versus no pamidronate in patients with primary breast cancer. Acta Oncol. 47, 740–746 (2008).
Diel, I. J. et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann. Oncol. 19, 2007–2011 (2008).
Diel, I. J. et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N. Engl. J. Med. 339, 357–363 (1998).
Powles, T. et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res. 8, R13 (2006).
Saarto, T., Vehmanen, L., Virkkunen, P. & Blomqvist, C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 43, 650–656 (2004).
Ha, T. C. & Li, H. Meta-analysis of clodronate and breast cancer survival. Br. J. Cancer 96, 1796–1801 (2007).
Gnant, M. et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N. Engl. J. Med. 360, 679–691 (2009).
Eidtmann, H. et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann. Oncol. doi:10.1093/annonc/mdq217.
Brufsky, A. M. et al. Zoledronic acid effectively prevents aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST study 36-month follow-up results. Clin. Breast Cancer 9, 77–85 (2009).
LLombarto, A. et al. Effect of zoledronic acid on aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: E-ZO-FAST 36-month follow up [abstract 231]. ASCO Meeting Abstracts 2009.
Costa, L. et al. Prospective evaluation of the peptide-bound collagen type I cross-links N-telopeptide and C-telopeptide in predicting bone metastases status. J. Clin. Oncol. 20, 850–856 (2002).
Brown, J. E. et al. Bone resorption predicts for skeletal complications in metastatic bone disease. Br. J. Cancer 89, 2031–2037 (2003).
Lipton, A. et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer 113, 193–201 (2008).
Woodward, J. K., Neville-Webbe, H. L., Coleman, R. E. & Holen, I. Combined effects of zoledronic acid and doxorubicin on breast cancer cell invasion in vitro. Anticancer Drugs 16, 845–854 (2005).
Ottewell, P. D. et al. Differential effect of doxorubicin and zoledronic acid on intraosseous versus extraosseous breast tumor growth in vivo. Clin. Cancer Res. 14, 4658–4666 (2008).
Coleman, R. E. et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br. J. Cancer 102, 1099–1105 (2010).
Aft, R. et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 11, 421–428 (2010).
Fournier, P. et al. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 62, 6538–6544 (2002).
Santini, D. et al. Changes in bone resorption and vascular endothelial growth factor after a single zoledronic acid infusion in cancer patients with bone metastases from solid tumours. Oncol. Rep. 15, 1351–1357 (2006).
Tas, F. et al. Effect of zoledronic acid on serum angiogenic factors in patients with bone metastases. Med. Oncol. 25, 346–349 (2008).
Boyle, W. J., Simonet, W. S. & Lacey, D. L. Osteoclast differentiation and activation. Nature 423, 337–342 (2003).
Stopeck, A. et al. A comparison of denosumab versus zoledronic acid for the prevention of skeletal-related events in breast cancer patients with bone metastases [abstract]. Cancer Res. 69 (Suppl. 3), 22 (2009).
Cummings, S. R. et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 361, 756–765 (2009).
Body, J. J. et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin. Cancer Res. 12, 1221–1228 (2006).
Cohen, S. B. et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 58, 1299–1309 (2008).
Thomas, D. et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 11, 275–280 (2010).
Ellis, G. K. et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J. Clin. Oncol. 26, 4875–4882 (2008).
Lipton, A. et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin. Cancer Res. 14, 6690–6696 (2008).
Lipton, A. et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J. Clin. Oncol. 25, 4431–4437 (2007).
Fizazi, K. et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J. Clin. Oncol. 27, 1564–1571 (2009).
Whyte, M. P. The long and the short of bone therapy. N. Engl. J. Med. 354, 860–863 (2006).
Canon, J. R. et al. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin. Exp. Metastasis 25, 119–129 (2008).
Rucci, N. et al. Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: implications for therapy. J. Pharmacol. Exp. Ther. 318, 161–172 (2006).
Hiscox, S., Jordan, N. J., Morgan, L., Green, T. P. & Nicholson, R. I. Src kinase promotes adhesion-independent activation of FAK and enhances cellular migration in tamoxifen-resistant breast cancer cells. Clin. Exp. Metastasis 24, 157–167 (2007).
Hiscox, S. et al. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res. Treat. 97, 263–274 (2006).
Thomas, S. M. & Brugge, J. S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13, 513–609 (1997).
Verbeek, B. S. et al. c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J. Pathol. 180, 383–388 (1996).
Summy, J. M. & Gallick, G. E. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 22, 337–358 (2003).
Metcalf, C. A. 3rd, van Schravendijk, M. R., Dalgarno, D. C. & Sawyer, T. K. Targeting protein kinases for bone disease: discovery and development of Src inhibitors. Curr. Pharm. Des. 8, 2049–2075 (2002).
Marzia, M. et al. Decreased c-Src expression enhances osteoblast differentiation and bone formation. J. Cell Biol. 151, 311–320 (2000).
Soriano, P., Montgomery, C., Geske, R. & Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702 (1991).
Horne, W. C. et al. Osteoclasts express high levels of pp60c-src in association with intracellular membranes. J. Cell Biol. 119, 1003–1013 (1992).
Sanjay, A. et al. Cbl associates with Pyk2 and Src to regulate Src kinase activity, alpha(v)beta(3) integrin-mediated signaling, cell adhesion, and osteoclast motility. J. Cell Biol. 152, 181–195 (2001).
Boyce, B. F., Yoneda, T., Lowe, C., Soriano, P. & Mundy, G. R. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J. Clin. Invest. 90, 1622–1627 (1992).
Myoui, A. et al. C-SRC tyrosine kinase activity is associated with tumor colonization in bone and lung in an animal model of human breast cancer metastasis. Cancer Res. 63, 5028–5033 (2003).
Zhang, X. H. et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 16, 67–78 (2009).
Hall, T. J., Schaeublin, M. & Missbach, M. Evidence that c-src is involved in the process of osteoclastic bone resorption. Biochem. Biophys. Res. Commun. 199, 1237–1244 (1994).
Yoneda, T. et al. Herbimycin A, a pp60c-src tyrosine kinase inhibitor, inhibits osteoclastic bone resorption in vitro and hypercalcemia in vivo. J. Clin. Invest. 91, 2791–2795 (1993).
Lombardo, L. J. et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J. Med. Chem. 47, 6658–6661 (2004).
Finn, R. S. et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res. Treat. 105, 319–326 (2007).
Huang, F. et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 67, 2226–2238 (2007).
Finn, R. S. et al. Phase II trial of dasatinib in triple-negative breast cancer: results of study CA180059 [abstract 3118]. SABCS Meeting Abstracts 2008.
de Vries, T. J. et al. The Src inhibitor AZD0530 reversibly inhibits the formation and activity of human osteoclasts. Mol. Cancer Res. 7, 476–488 (2009).
Hiscox, S. et al. Dual targeting of Src and ER prevents acquired antihormone resistance in breast cancer cells. Breast Cancer Res. Treat. 115, 57–67 (2009).
Chen, Y. et al. Combined Src and aromatase inhibition impairs human breast cancer growth in vivo and bypass pathways are activated in AZD0530-resistant tumors. Clin. Cancer Res. 15, 3396–3405 (2009).
Herynk, M. H. et al. Cooperative action of tamoxifen and c-Src inhibition in preventing the growth of estrogen receptor-positive human breast cancer cells. Mol. Cancer Ther. 5, 3023–3031 (2006).
Jallal, H. et al. A Src/Abl kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo. Cancer Res. 67, 1580–1588 (2007).
Vultur, A. et al. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol. Cancer Ther. 7, 1185–1194 (2008).
Drake, F. H. et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J. Biol. Chem. 271, 12511–12516 (1996).
Silver, I. A., Murrills, R. J. & Etherington, D. J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 175, 266–276 (1988).
Garnero, P. et al. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J. Biol. Chem. 273, 32347–32352 (1998).
Gowen, M. et al. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J. Bone Miner. Res. 14, 1654–1663 (1999).
Saftig, P. et al. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc. Natl Acad. Sci. USA 95, 13453–13458 (1998).
Le Gall, C. et al. A cathepsin K inhibitor reduces breast cancer induced osteolysis and skeletal tumor burden. Cancer Res. 67, 9894–9902 (2007).
Adami, S. et al. Effect of one year treatment with the cathepsin-K inhibitor, balicatib, on bone mineral density in postmenopausal women with osteopenia/osteoporosis [abstract]. J. Bone Miner. Res. 21 (Suppl. 1), 1085 (2006).
Peroni, A. et al. Drug-induced morphea: report of a case induced by balicatib and review of the literature. J. Am. Acad. Dermatol. 59, 125–129 (2008).
Falgueyret, J. P. et al. Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off-target cathepsins and reduced functional selectivity. J. Med. Chem. 48, 7535–7543 (2005).
Khalfan, H. A. Study of thiol proteases of normal human skin fibroblasts. Cell Biochem. Funct. 9, 55–62 (1991).
Bone, H. G. et al. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J. Bone Miner. Res. 25, 937–947 (2010).
Ramirez, G. et al. Effect of cathepsin K inhibition on suppression of bone resorption in women with breast cancer and established bone metastases in a 4-week, double-blind, randomized controlled trial [abstract 209]. ASCO Breast Cancer Symposium 2008.
Guise, T. A. et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Invest. 98, 1544–1549 (1996).
Guise, T. A. et al. Molecular mechanisms of breast cancer metastases to bone. Clin. Breast Cancer 5 (Suppl. 2), S46–S53 (2005).
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Linforth, R. et al. Coexpression of parathyroid hormone related protein and its receptor in early breast cancer predicts poor patient survival. Clin. Cancer Res. 8, 3172–3177 (2002).
Henderson, M. A. et al. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res. 66, 2250–2256 (2006).
Yin, J. J. et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 103, 197–206 (1999).
Kang, Y. et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl Acad. Sci. USA 102, 13909–13914 (2005).
Ishida, A., Fujita, N., Kitazawa, R. & Tsuruo, T. Transforming growth factor-beta induces expression of receptor activator of NF-kappa B ligand in vascular endothelial cells derived from bone. J. Biol. Chem. 277, 26217–26224 (2002).
Sanchez-Sweatman, O. H., Lee, J., Orr, F. W. & Singh, G. Direct osteolysis induced by metastatic murine melanoma cells: role of matrix metalloproteinases. Eur. J. Cancer 33, 918–925 (1997).
Duivenvoorden, W. C., Hirte, H. W. & Singh, G. Transforming growth factor beta1 acts as an inducer of matrix metalloproteinase expression and activity in human bone-metastasizing cancer cells. Clin. Exp. Metastasis 17, 27–34 (1999).
de Jong, J. S., van Diest, P. J., van der Valk, P. & Baak, J. P. Expression of growth factors, growth-inhibiting factors, and their receptors in invasive breast cancer. II: Correlations with proliferation and angiogenesis. J. Pathol. 184, 53–57 (1998).
Miettinen, P. J., Ebner, R., Lopez, A. R. & Derynck, R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J. Cell Biol. 127, 2021–2036 (1994).
Beck, C., Schreiber, H. & Rowley, D. Role of TGF-beta in immune-evasion of cancer. Microsc. Res. Tech. 52, 387–395 (2001).
Derynck, R., Akhurst, R. J. & Balmain, A. TGF-beta signaling in tumor suppression and cancer progression. Nat. Genet. 29, 117–129 (2001).
Shi, Y. & Massagué, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685–700 (2003).
Massagué, J. TGFbeta in cancer. Cell 134, 215–230 (2008).
Mourskaia, A. A. et al. Transforming growth factor-beta1 is the predominant isoform required for breast cancer cell outgrowth in bone. Oncogene 28, 1005–1015 (2009).
Ehata, S. et al. Ki26894, a novel transforming growth factor-beta type I receptor kinase inhibitor, inhibits in vitro invasion and in vivo bone metastasis of a human breast cancer cell line. Cancer Sci. 98, 127–133 (2007).
Bandyopadhyay, A. et al. Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-beta type I receptor kinase inhibitor. Cancer Res. 66, 6714–6721 (2006).
Oettle, H. et al. Interim results of the phase I/II study of trabedersen (AP 12009) in patients with pancreatic carcinoma, malignant melanoma, or colorectal carcinoma [abstract 4619]. ASCO Meeting Abstracts 2009.
Bogdahn, U. et al. Randomized, active-controlled phase IIb study with trabedersen (AP 12009) in recurrent or refractory high-grade glioma patients: Basis for phase III endpoints [abstract 2037]. ASCO Meeting Abstracts 2009.
Nemunaitis, J. et al. Phase II trial of Belagenpumatucel-L, a TGF-beta2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther. 16, 620–624 (2009).
Morris, J. C. et al. Phase I/II study of GC1008: a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody (MAb) in patients with advanced malignant melanoma (MM) or renal cell carcinoma (RCC) [abstract 9028]. ASCO Meeting Abstracts 2008.
Tan, A. R., Alexe, G. & Reiss, M. Transforming growth factor-beta signaling: emerging stem cell target in metastatic breast cancer? Breast Cancer Res. Treat. 115, 453–495 (2009).
Nagaraj, N. S. & Datta, P. K. Targeting the transforming growth factor-beta signaling pathway in human cancer. Expert Opin. Investig. Drugs 19, 77–91 (2010).
Padua, D. et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133, 66–77 (2008).
Baselga, J. et al. TGF-beta signalling-related markers in cancer patients with bone metastasis. Biomarkers 13, 217–236 (2008).
Müller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001).
Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 4, 540–550 (2004).
Cabioglu, N. et al. Chemokine receptors in advanced breast cancer: differential expression in metastatic disease sites with diagnostic and therapeutic implications. Ann. Oncol. 20, 1013–1019 (2009).
Peled, A. et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283, 845–848 (1999).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Richert, M. M. et al. Inhibition of CXCR4 by CTCE-9908 inhibits breast cancer metastasis to lung and bone. Oncol. Rep. 21, 761–767 (2009).
Huang, E. H. et al. A CXCR4 antagonist CTCE-9908 inhibits primary tumor growth and metastasis of breast cancer. J. Surg. Res. 155, 231–236 (2009).
Hotte, S. J. et al. Final results of a phase I/II study of CTCE-9908, a novel anticancer agent that inhibits CXCR4, in patients with advanced solid cancers [abstract 405]. 20th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics 2008.
Brave, M. et al. FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology 78, 282–288 (2010).
Denoyelle, C., Hong, L., Vannier, J. P., Soria, J. & Soria, C. New insights into the actions of bisphosphonate zoledronic acid in breast cancer cells by dual RhoA-dependent and -independent effects. Br. J. Cancer 88, 1631–1640 (2003).
Tu, S. M. et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet 357, 336–341 (2001).
Palmedo, H. et al. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: randomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. J. Clin. Oncol. 21, 2869–2875 (2003).
Amato, R. J., Hernandez-McClain, J. & Henary, H. Bone-targeted therapy: phase II study of strontium-89 in combination with alternating weekly chemohormonal therapies for patients with advanced androgen-independent prostate cancer. Am. J. Clin. Oncol. 31, 532–538 (2008).
Ueno, N. T. et al. Pilot study of targeted skeletal radiation therapy for bone-only metastatic breast cancer. Clin. Breast Cancer 9, 173–177 (2009).
Hamaoka, T., Madewell, J. E., Podoloff, D. A., Hortobagyi, G. N. & Ueno, N. T. Bone imaging in metastatic breast cancer. J. Clin. Oncol. 22, 2942–2953 (2004).
Bäuerle, T. & Semmler, W. Imaging response to systemic therapy for bone metastases. Eur. Radiol. 19, 2495–2507 (2009).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005).
Cristofanilli, M. et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J. Clin. Oncol. 23, 1420–1430 (2005).
De Giorgi, U. et al. Circulating tumor cells and bone metastases as detected by FDG-PET/CT in patients with metastatic breast cancer. Ann. Oncol. 21, 33–39 (2010).
Smid, M. et al. Genes associated with breast cancer metastatic to bone. J. Clin. Oncol. 24, 2261–2267 (2006).
Wang, Y. et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365, 671–679 (2005).
Woelfle, U. et al. Molecular signature associated with bone marrow micrometastasis in human breast cancer. Cancer Res. 63, 5679–5684 (2003).
Smith, M. R. et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 361, 745–755 (2009).
Acknowledgements
This work was supported in part by the NIH Cancer Center Support Grant CA016672, and by the Nellie B. Connally Breast Cancer Research Fund. We thank Sunita Patterson (Department of Scientific Publications at The University of Texas MD Anderson Cancer Center) for editorial assistance. C. P. Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the MedscapeCME-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Contributions
T. Onishi and N. T. Ueno contributed to discussion of content for the article, researched data to include in the manuscript, wrote the content, reviewed and edited the manuscript before submission, and revised the manuscript in response to the peer reviewers' comments. N. Hayashi researched data to include in the manuscript and contributed to the writing. R. L. Theriault and G. N. Hortobagyi reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
G. N. Hortobagyi has worked as a consultant for Merck and Sanofi-Aventis, and has received a grant/research support from and worked as a consultant for Novartis. N. T. Ueno has received a grant/research support from EUSA Pharma. T. Onishi, N. Hayashi, and R. L. Theriault declare no competing interests.
Rights and permissions
About this article
Cite this article
Onishi, T., Hayashi, N., Theriault, R. et al. Future directions of bone-targeted therapy for metastatic breast cancer. Nat Rev Clin Oncol 7, 641–651 (2010). https://doi.org/10.1038/nrclinonc.2010.134
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2010.134
This article is cited by
-
New insights into breast microcalcification for poor prognosis: NACT cohort and bone metastasis evaluation cohort
Journal of Cancer Research and Clinical Oncology (2023)
-
Application of additively manufactured 3D scaffolds for bone cancer treatment: a review
Bio-Design and Manufacturing (2022)
-
Novel approaches to target the microenvironment of bone metastasis
Nature Reviews Clinical Oncology (2021)
-
The genomic architecture of metastasis in breast cancer: focus on mechanistic aspects, signalling pathways and therapeutic strategies
Medical Oncology (2021)
-
Randomized phase-II evaluation of letrozole plus dasatinib in hormone receptor positive metastatic breast cancer patients
npj Breast Cancer (2019)