Abstract
A subset of patients with chronic myeloid leukemia (CML) who receive imatinib therapy will require alternative therapy at some point owing to safety reasons or lack of efficacy. Achieving an early response with imatinib is protective against treatment failure; second-generation tyrosine kinase inhibitors (TKIs; for example, nilotinib, dasatinib, bosutinib), however, have proven to be efficacious at restoring cytogenetic responses in patients who require subsequent therapy. Response duration, however, is yet to be established and a considerable proportion of patients fail to achieve a clinically meaningful response. A third generation of TKIs is currently undergoing clinical testing for use in patients who fail imatinib and a second-generation TKI. Most of these agents are multikinase inhibitors with activity against a wide variety of BCR-ABL1 mutations, including the highly resistant T315I. The use of second-generation TKIs in the frontline setting seems to provide higher rates of early response compared with imatinib. If these results are confirmed in randomized studies, nilotinib and dasatinib could replace imatinib as standard frontline therapy in CML. Despite the activity of all of the above mentioned agents, curing CML will ultimately depend on the development of agents capable of vanquishing BCR-ABL1-positive CML stem cells. Efforts aimed at achieving this goal are ongoing.
Key Points
-
The phase III IRIS trial established imatinib as standard therapy for chronic phase CML; an update indicates that a substantial proportion of patients will require alternative therapy during the course of treatment
-
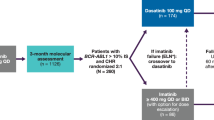
The second-generation tyrosine kinase inhibitors nilotinib and dasatinib can restore cytogenetic response in patients who have failed imatinib therapy; response duration, however, has not been fully established
-
Nilotinib or dasatinib therapy in the frontline setting seems to provide rapid and improved rates of cytogenetic and molecular response, which could potentially translate into improved long-term outcomes
-
Imatinib discontinuation upon achievement of complete molecular response results in high rates of relapse, probably because of the innate insensitivity of CML stem cells to tyrosine kinase inhibitors
-
There is an urgent need to identify pharmacologic inhibitors of pathways essential for the maintenance of BCR-ABL1-positive leukemic initiating cells
-
Several agents with activity against the highly insensitive BCR-ABL1 T315I mutation are being evaluated in clinical trials
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Druker, B. J. et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 355, 2408–2417 (2006).
O'Brien, S. G. et al. International Randomized Study of Interferon Versus STI571 (IRIS) 7-year follow-up: sustained survival, low rate of transformation and increased rate of major molecular response (MMR) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib (IM) [abstract]. Blood 112, 186 (2008).
Hughes, T. P. et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 349, 1423–1432 (2003).
Cortes, J. et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin. Cancer Res. 11, 3425–3432 (2005).
Quintas-Cardama, A. & Cortes, J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood 113, 1619–1630 (2009).
Druker, B. J. et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344, 1038–1042 (2001).
Hughes, T. et al. Reduction of BCR-ABL transcript levels at 6, 12, and 18 months (mo) correlates with long-term outcomes on imatinib (IM) at 72 mo: An analysis from the international randomized study of interferon versus STI571 (IRIS) in patients (pts) with chronic phase chronic myeloid leukemia (CML-CP) [abstract]. Blood 11, 334 (2008).
de Lavallade, H. et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J. Clin. Oncol. 26, 3358–3363 (2008).
Press, R. D. et al. A half-log increase in BCR-ABL RNA predicts a higher risk of relapse in patients with chronic myeloid leukemia with an imatinib-induced complete cytogenetic response. Clin. Cancer Res. 13, 6136–6143 (2007).
Branford, S. et al. BCR-ABL messenger RNA levels continue to decline in patients with chronic phase chronic myeloid leukemia treated with imatinib for more than 5 years and approximately half of all first-line treated patients have stable undetectable BCR-ABL using strict sensitivity criteria. Clin. Cancer Res. 13, 7080–7085 (2007).
Iacobucci, I. et al. Comparison between patients with Philadelphia-positive chronic phase chronic myeloid leukemia who obtained a complete cytogenetic response within 1 year of imatinib therapy and those who achieved such a response after 12 months of treatment. J. Clin. Oncol. 24, 454–459 (2006).
Guilhot, F. et al. Time to complete cytogenetic response (CCyR) does not affect long-term outcomes for patients on imatinib therapy [abstract]. Blood 110, 27 (2007).
Quintas-Cardama, A., Kantarjian, H. & Cortes, J. Tyrosine kinase inhibitors for chronic myelogenous leukemia. N. Engl. J. Med. 357, 1557 (2007).
Quintas-Cardama, A. et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myelogenous leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood 113, 6315–6321 (2009).
Kantarjian, H. et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood 103, 2873–2878 (2004).
Cortes, J. et al. Result of high-dose imatinib mesylate in patients with Philadelphia chromosome-positive chronic myeloid leukemia after failure of interferon-alpha. Blood 102, 83–86 (2003).
Hughes, T. P. et al. Impact of early dose intensity on cytogenetic and molecular responses in chronic- phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood 112, 3965–3973 (2008).
Larson, R. A. et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood 111, 4022–4028 (2008).
Picard, S. et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 109, 3496–3499 (2007).
Guilhot, F. et al. Imatinib (IM) pharmacokinetic (PK) exposure and its correlation with clinical outcome in patients with chronic-phase chronic myeloid leukemia (CML-CP) for 400 mg and 800 mg daily doses (Tyrosine Kinase Dose Optimization Study [TOPS]) [abstract]. Blood 112, 447 (2008).
Ault, P. et al. Clinical use of imatinib plasma levels in patients with chronic myeloid leukemia (CML) [abstract]. Blood 112, 4255 (2008).
Cortes, J. et al. A phase III, randomized, open-label study of 400 mg versus 800 mg of imatinib mesylate (IM) in patients (pts) with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase (CML-CP) using molecular endpoints: 1-year results of TOPS (Tyrosine Kinase Inhibitor Optimization and Selectivity) study [abstract]. Blood 112, 335 (2008).
Baccarani, M. et al. Cytogenetic and molecular response to imatinib in high risk (Sokal) chronic myeloid leukemia (CML): results of an European Leukemianet prospective study comparing 400 mg and 800 mg front-line [abstract]. Blood 112, 185 (2008).
Rosti, G. et al. High and early rates of cytogenetic and molecular response with nilotinib 800 mg daily as first line treatment of Ph-positive chronic myeloid leukemia in chronic phase: results of a phase 2 trial of the GIMEMA CML Working Party [abstract]. Blood 112, 181 (2008).
Cortes, J. et al. Efficacy of dasatinib in patients (pts) with previously untreated chronic myelogenous leukemia (CML) in early chronic phase (CML-CP) [abstract]. Blood 112, 182 (2008).
Cortes, J. et al. Efficacy of nilotinib (formerly AMN107) in patients (pts) with newly diagnosed, previously untreated Philadelphia chromosome (Ph)-positive chronic myelogenous leukemia in early chronic phase (CML-CP) [abstract]. Blood 112, 446 (2008).
Holtz, M. S., Forman, S. J. & Bhatia, R. Nonproliferating CML CD34+ progenitors are resistant to apoptosis induced by a wide range of proapoptotic stimuli. Leukemia 19, 1034–1041 (2005).
Holtz, M. S. et al. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood 99, 3792–3800 (2002).
Graham, S. M. et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 99, 319–325 (2002).
Rousselot, P. et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood 109, 58–60 (2007).
Mahon, F. et al. Is it possible to stop imatinib in patients with chronic myeloid leukemia? An update from a French pilot study and first results from the multicentre 'Stop Imatinib' (STIM) study [abstract]. Blood 112, 187 (2008).
Verma, D., Kantarjian, H., Jain, N. & Cortes, J. Sustained complete molecular response after imatinib discontinuation in a patient with chronic myeloid leukemia not previously exposed to interferon alpha. Leuk. Lymphoma 49, 1399–1402 (2008).
Copland, M. et al. BMS-214662 potently induces apoptosis of chronic myeloid leukemia stem and progenitor cells and synergizes with tyrosine kinase inhibitors. Blood 111, 2843–2853 (2008).
Strauss, A. et al. Effective induction of apoptosis in chronic myeloid leukemia CD34+ cells by the histone deacetylase inhibitor LAQ824 in combination with imatinib [abstract]. Blood 110, 1031 (2007).
Ito, K. et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature 453, 1072–1078 (2008).
Zhao, C. et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 458, 776–779 (2009).
Bradeen, H. A. et al. Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: high efficacy of drug combinations. Blood 108, 2332–2338 (2006).
Quintas-Cardama, A., Kantarjian, H. & Cortes, J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat. Rev. Drug Discov. 6, 834–848 (2007).
Carter, T. A. et al. Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc. Natl Acad. Sci. USA 102, 11011–11016 (2005).
Cortes, J. et al. Preliminary clinical activity in a phase I trial of the BCR-ABL/IGF-1R/aurora kinase inhibitor XL228 in patients with Ph+ leukemias with either failure to multiple TKI therapies or with T315I mutation [abstract]. Blood 112, 3232 (2008).
Paquette, R. et al. PHA-739358, an aurora kinase inhibitor, induces clinical responses in chronic myeloid leukemia harboring T315I mutations of BCR-ABL. Blood 110, 1030 (2007).
Van Etten, R. A. et al. Switch pocket inhibitors of the ABL tyrosine kinase: distinct kinome inhibition profiles and in vivo efficacy in mouse models of CML and B-lymphoblastic leukemia induced by BCR-ABL T315I [abstract]. Blood 112, 576 (2008).
Kantarjian, H. M. et al. Homoharringtonine: history, current research, and future direction. Cancer 92, 1591–1605 (2001).
Kozopas, K. M., Yang, T., Buchan, H. L., Zhou, P. & Craig, R. W. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl Acad. Sci. USA 90, 3516–3520 (1993).
Tang, R. et al. Semisynthetic homoharringtonine induces apoptosis via inhibition of protein synthesis and triggers rapid myeloid cell leukemia-1 down-regulation in myeloid leukemia cells. Mol. Cancer Ther. 5, 723–731 (2006).
Chen, Y. et al. Inhibitory effects of homoharringtonine on leukemic stem cells and BCR-ABL induced chronic myeloid leukemia and acute lymphoblastic leukemia in mice [abstract]. Blood 110, 2912 (2007).
Cortes, J. et al. Safety and efficacy of subcutaneous (SC) omacetaxine mepesuccinate in imatinib(IM)-resistant chronic myeloid leukemia (CML) patients (pts) with the T315I mutation—results of an ongoing multicenter phase II study [abstract]. Blood 112, 3239 (2008).
Hochhaus, A. et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia 22, 1200–1206 (2008).
Guilhot, F. et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood 109, 4143–4150 (2007).
Cortes, J. et al. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase. Leukemia 22, 2176–2183 (2008).
Kantarjian, H. et al. Nilotinib in chronic myeloid leukemia patients in chronic phase (CML-CP) with imatinib resistance or intolerance: 2-year follow-up results of a phase 2 study [abstract]. Blood 112, 3238 (2008).
Le Coutre, P. et al. Nilotinib in chronic myeloid leukemia patients in accelerated phase (CML-AP) with imatinib resistance or intolerance: 2-year follow-up results of a phase 2 study [abstract]. Blood 112, 3229 (2008).
Giles, F. et al. Nilotinib (Tasigna) in pts with Ph+ CML-BC who are resistant or intolerant to imatinib [abstract]. Blood 110, 1025 (2007).
Cortes, J. et al. Efficacy and safety of bosutinib (SKI-606) in patients with chronic phase (CP) Ph+ chronic myelogenous leukemia (CML) with resistance or intolerance to imatinib [abstract]. Blood 112, 1098 (2008).
Gambacorti-Passerini, C. et al. Bosutinib (SKI-606) demonstrates clinical activity and is well tolerated in patients with AP and BP CML and Ph+ ALL [abstract]. Blood 112, 1101 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J. Cortes declares he receives grant/research support from BMS, Novartis, and Wyeth. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Quintás-Cardama, A., Kantarjian, H. & Cortes, J. Imatinib and beyond—exploring the full potential of targeted therapy for CML. Nat Rev Clin Oncol 6, 535–543 (2009). https://doi.org/10.1038/nrclinonc.2009.112
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2009.112
This article is cited by
-
Mutation analysis of BCR-ABL1 kinase domain in chronic myeloid leukemia patients with tyrosine kinase inhibitors resistance: a Malaysian cohort study
BMC Research Notes (2024)
-
Inhibition of LATS kinases reduces tumorigenicity and increases the sensitivity of human chronic myelogenous leukemia cells to imatinib
Scientific Reports (2024)
-
In Vitro Cytotoxicity of Ferula asafoetida Gum Extract on Human Chronic Myelogenous Leukemia K562 Cells
Pharmaceutical Chemistry Journal (2022)
-
Tyrosine Kinase Inhibition: a New Perspective in the Fight against HIV
Current HIV/AIDS Reports (2019)
-
c-Abl regulates gastrointestinal muscularis propria homeostasis via ERKs
Scientific Reports (2017)