Key Points
-
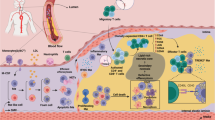
The mononuclear phagocyte system comprises heterogeneous cells derived from fetal and bone marrow precursors
-
Seeding of fetal tissue macrophages is distinct from monocyte-derived macrophages, highlighting the independent effector functions of monocyte subsets
-
Dyslipidaemia, characterized by hypercholesterolaemia and/or hypertriglyceridaemia, increases the risk of cardiovascular disease
-
Dyslipidaemia increases the production of monocytes through myelopoiesis
-
Blood monocyte subsets under dyslipidaemic conditions change their behaviour and activation, and increase their extravasation in response to triglyceride-rich lipoproteins and cholesterol
Abstract
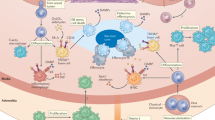
Monocytes are heterogeneous effector cells involved in the maintenance and restoration of tissue integrity. Monocytes and macrophages are involved in cardiovascular disease progression, and are associated with the development of unstable atherosclerotic plaques. Hyperlipidaemia can accelerate cardiovascular disease progression. However, monocyte responses to hyperlipidaemia are poorly understood. In the past decade, accumulating data describe the relationship between the dynamic blood lipid environment and the heterogeneous circulating monocyte pool, which might have profound consequences for cardiovascular disease. In this Review, we explore the updated view of monocytes in cardiovascular disease and their relationship with macrophages in promoting the homeostatic and inflammatory responses related to atherosclerosis. We describe the different definitions of dyslipidaemia, highlight current theories on the ontogeny of monocyte heterogeneity, discuss how dyslipidaemia might alter monocyte production, and explore the mechanistic interface linking dyslipidaemia with monocyte effector functions, such as migration and the inflammatory response. Finally, we discuss the role of dietary and endogenous lipid species in mediating dyslipidaemic responses, and the role of these lipids in promoting the risk of cardiovascular disease through modulation of monocyte behaviour.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ginhoux, F. & Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014).
Robbins, C. S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
Cros, J. et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33, 375–386 (2010).
van Furth, R. & Cohn, Z. A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 128, 415–435 (1968).
Shi, C. & Pamer, E. G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11, 762–774 (2011).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Takahashi, K., Yamamura, F. & Naito, M. Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J. Leukoc. Biol. 45, 87–96 (1989).
Takahashi, K. & Naito, M. Development, differentiation, and proliferation of macrophages in the rat yolk sac. Tissue Cell 25, 351–362 (1993).
Palis, J. et al. Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc. Natl Acad. Sci. USA 98, 4528–4533 (2001).
McGrath, K. E., Koniski, A. D., Malik, J. & Palis, J. Circulation is established in a stepwise pattern in the mammalian embryo. Blood 101, 1669–1676 (2003).
Orkin, S. H. & Zon, L. I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–644 (2008).
Guilliams, M. et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 210, 1977–1992 (2013).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Epelman, S. et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 (2014).
Hoeffel, G. et al. C-Myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678 (2015).
Hoeffel, G. et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 209, 1167–1181 (2012).
Jaensson, E. et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 205, 2139–2149 (2008).
Bain, C. C. et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 15, 929–937 (2014).
Cecchini, M. G. et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development 120, 1357–1372 (1994).
Dai, X.-M. et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99, 111–120 (2002).
Wiktor-Jedrzejczak, W. & Gordon, S. Cytokine regulation of the macrophage (M phi) system studied using the colony stimulating factor-1-deficient op/op mouse. Physiol. Rev. 76, 927–947 (1996).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Robbins, C. S. et al. Extramedullary hematopoiesis generates Ly-6Chigh monocytes that infiltrate atherosclerotic lesionsclinical perspective. Circulation 125, 364–374 (2012).
Fogg, D. K. et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311, 83–87 (2006).
Sathe, P. et al. Lymphoid tissue and plasmacytoid dendritic cells and macrophages do not share a common macrophage-dendritic cell-restricted progenitor. Immunity 41, 104–115 (2014).
Hettinger, J. et al. Origin of monocytes and macrophages in a committed progenitor. Nat. Immunol. 14, 821–830 (2013).
Imhof, B. A. & Aurrand-Lions, M. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 4, 432–444 (2004).
Volkman, A. The origin and fate of the monocyte. Ser. Haematol. 3, 62–92 (1970).
Vu Manh, T.-P. et al. Defining mononuclear phagocyte subset homology across several distant warm-blooded vertebrates through comparative transcriptomics. Front. Immunol. 6, 299 (2015).
Etzrodt, M. et al. Regulation of monocyte functional heterogeneity by miR-146a and Relb. Cell Rep. 1, 317–324 (2012).
Ingersoll, M. A. et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 115, e10–e19 (2010).
Mildner, A. et al. Mononuclear phagocyte miRNome analysis identifies miR-142 as critical regulator of murine dendritic cell homeostasis. Blood 121, 1016–1027 (2013).
Douglas, S. D. & Douglas, A. G. in Williams Manual of Hematology (eds Kaushansky, K., Beutler, E., Seligsohn, U., Lichtman, M. A. & Prchal, J. T.) 1045–1073 (McGraw-Hill Professional, 2011).
Ziegler-Heitbrock, L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J. Leukoc. Biol. 81, 584–592 (2007).
Ancuta, P. et al. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16- monocyte subsets. BMC Genomics 10, 403 (2009).
Auffray, C. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670 (2007).
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Sunderkötter, C. et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172, 4410–4417 (2004).
Zhao, C. et al. Identification of novel functional differences in monocyte subsets using proteomic and transcriptomic methods. J. Proteome Res. 8, 4028–4038 (2009).
Yona, S. & Jung, S. Monocytes: subsets, origins, fates and functions. Curr. Opin. Hematol. 17, 53–59 (2010).
Ziegler-Heitbrock, L. Monocyte subsets in man and other species. Cell. Immunol. 289, 135–139 (2014).
Woollard, K. J. & Geissmann, F. Monocytes in atherosclerosis: subsets and functions. Nat. Rev. Cardiol. 7, 77–86 (2010).
Randolph, G. J. Mechanisms that regulate macrophage burden in atherosclerosis. Circ. Res. 114, 1757–1771 (2014).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Palframan, R. T. et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194, 1361–1374 (2001).
Jakubzick, C. et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 39, 599–610 (2013).
Moore, K. J., Sheedy, F. J. & Fisher, E. A. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13, 709–721 (2013).
Wong, K. L. et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 118, e16–e31 (2011).
Rogacev, K. S. et al. Lower Apo A-I and lower HDL-C levels are associated with higher intermediate CD14++CD16+ monocyte counts that predict cardiovascular events in chronic kidney disease. Arterioscler. Thromb. Vasc. Biol. 34, 2120–2127 (2014).
Mukherjee, R. et al. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci. Rep. 5, 13886 (2015).
Ziegler-Heitbrock, L. & Hofer, T. P. J. Toward a refined definition of monocyte subsets. Front. Immunol. 4, 23 (2013).
Hilgendorf, I. et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ. Res. 114, 1611–1622 (2014).
Hanna, R. N. et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C− monocytes. Nat. Immunol. 12, 778–785 (2011).
Carlin, L. M. et al. Nr4a1-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. Cell 153, 362–375 (2013).
Hanna, R. N. et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ. Res. 110, 416–427 (2012).
Hamers, A. A. J. et al. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ. Res. 110, 428–438 (2012).
Chao, L. C. et al. Bone marrow NR4A expression is not a dominant factor in the development of atherosclerosis or macrophage polarization in mice. J. Lipid Res. 54, 806–815 (2013).
Chong, S. Z. et al. CXCR4 identifies transitional bone marrow premonocytes that replenish the mature monocyte pool for peripheral responses. J. Exp. Med. 213, 2293–2314 (2016).
Menezes, S. et al. The heterogeneity of Ly6Chi monocytes controls their differentiation into iNOS+ macrophages or monocyte-derived dendritic cells. Immunity 45, 1205–1218 (2016).
Satoh, T. et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature 541, 96–101 (2017).
Jackson, W. D., Weinrich, T. W. & Woollard, K. J. Very-low and low-density lipoproteins induce neutral lipid accumulation and impair migration in monocyte subsets. Sci. Rep. 6, 20038 (2016).
Saja, M. F. et al. Triglyceride-rich lipoproteins modulate the distribution and extravasation of Ly6C/Gr1low monocytes. Cell Rep. 12, 1802–1815 (2015).
Misharin, A. V. et al. Nonclassical Ly6C− monocytes drive the development of inflammatory arthritis in mice. Cell Rep. 9, 591–604 (2014).
Sampson, M. J., Davies, I. R., Brown, J. C., Ivory, K. & Hughes, D. A. Monocyte and neutrophil adhesion molecule expression during acute hyperglycemia and after antioxidant treatment in type 2 diabetes and control patients. Arterioscler. Thromb. Vasc. Biol. 22, 1187–1193 (2002).
Imhof, B. A. et al. CCN1/CYR61-mediated meticulous patrolling by Ly6Clow monocytes fuels vascular inflammation. Proc. Natl Acad. Sci. USA 113, E4847–E4856 (2016).
Finsterbusch, M. et al. Patrolling monocytes promote intravascular neutrophil activation and glomerular injury in the acutely inflamed glomerulus. Proc. Natl Acad. Sci. USA 113, E5172–E5181 (2016).
Michaud, J.-P., Bellavance, M.-A., Préfontaine, P. & Rivest, S. Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep. 5, 646–653 (2013).
Hanna, R. N. et al. Patrolling monocytes control tumor metastasis to the lung. Science 350, 985–990 (2015).
Li, W. et al. Intravital 2-photon imaging of leukocyte trafficking in beating heart. J. Clin. Invest. 122, 2499–2508 (2012).
Murphy, A. J. et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 121, 4138–4149 (2011).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Tolani, S. et al. Hypercholesterolemia and reduced HDL-C promote hematopoietic stem cell proliferation and monocytosis: studies in mice and FH children. Atherosclerosis 229, 79–85 (2013).
Yvan-Charvet, L., Wang, N. & Tall, A. R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 30, 139–143 (2010).
Yvan-Charvet, L. et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328, 1689–1693 (2010).
Chapman, C. M. L., Beilby, J. P., McQuillan, B. M., Thompson, P. L. & Hung, J. Monocyte count, but not C-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke 35, 1619–1624 (2004).
Johnsen, S. H. et al. Monocyte count is a predictor of novel plaque formation: a 7-year follow-up study of 2610 persons without carotid plaque at baseline the Tromsø Study. Stroke 36, 715–719 (2005).
Ganda, A. et al. Mild renal dysfunction and metabolites tied to low HDL cholesterol are associated with monocytosis and atherosclerosis. Circulation 127, 988–996 (2013).
Combadière, C. et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117, 1649–1657 (2008).
Swirski, F. K. et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc. Natl Acad. Sci. USA 103, 10340–10345 (2006).
Lessner, S. M., Prado, H. L., Waller, E. K. & Galis, Z. S. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am. J. Pathol. 160, 2145–2155 (2002).
Landsman, L. et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 113, 963–972 (2009).
Potteaux, S. et al. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J. Clin. Invest. 121, 2025–2036 (2011).
Murphy, A. J. & Tall, A. R. Proliferating macrophages populate established atherosclerotic lesions. Circ. Res. 114, 236–238 (2014).
Psaltis, P. J. et al. Characterization of a resident population of adventitial macrophage progenitor cells in postnatal vasculature. Circ. Res. 115, 364–375 (2014).
Ensan, S. et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1+ precursors and circulating monocytes immediately after birth. Nat. Immunol. 17, 159–168 (2016).
Dutta, P. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012).
Heidt, T. et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ. Res. 115, 284–295 (2014).
Fodor, G. Primary prevention of CVD: treating dyslipidaemia. BMJ Clin. Evid. 2010, 0215 (2010).
Prospective Studies Collaboration et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 370, 1829–1839 (2007).
Karpe, F., Dickmann, J. R. & Frayn, K. N. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 60, 2441–2449 (2011).
Hansson, G. K. & Libby, P. The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 6, 508–519 (2006).
Freigang, S. et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat. Immunol. 14, 1045–1053 (2013).
Fredrickson, D. S. & Lees, R. S. A. System for phenotyping hyperlipoproteinemia. Circulation 31, 321–327 (1965).
Beaumont, J. L. et al. Classification of hyperlipidaemias and hyperlipoproteinaemias. Bull. World Health Organ. 43, 891–915 (1970).
Catapano, A. L. et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur. Heart J. 37, 2999–3058 (2016).
Stone, N. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation 129, S1–S45 (2014).
Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283 (2013).
Brown, M. S. & Goldstein, J. L. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc. Natl Acad. Sci. USA 71, 788–792 (1974).
Fredrickson, D. S. & Levy, R. I. in The Metabolic Basis of Inherited Disease Vol. 3 (eds Stanbury, J. B., Fredrickson, D. S. & Wyngaarden, J. B.) 544–589 (McGraw-Hill, 1978).
Vogel, J., Day, G. E., Cantab, L. M. & Baillière, H. The Pathological Anatomy of the Human Body (H. Bailliere, 1847).
Anitschkow, N. Die pathologischen Veränderungen innerer Organe bei experimenteller Cholesterinesterverfettung. Dtsch Med. Wochenschr. 39, 741–743 (in German) (2009).
Levy, R. I. et al. Cholestyramine in type II hyperlipoproteinemia. A double-blind trial. Ann. Intern. Med. 79, 51–58 (1973).
Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344, 1383–1389 (1994).
Shepherd, J. et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 333, 1301–1307 (1995).
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360, 7–22 (2002).
Cannon, C. P. et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 350, 1495–1504 (2004).
Cannon, C. P. et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 372, 2387–2397 (2015).
Jovinge, S., Ares, M. P., Kallin, B. & Nilsson, J. Human monocytes/macrophages release TNF-alpha in response to Ox-LDL. Arterioscler. Thromb. Vasc. Biol. 16, 1573–1579 (1996).
Terkeltaub, R. et al. Oxidized LDL induces monocytic cell expression of interleukin-8, a chemokine with T-lymphocyte chemotactic activity. Arterioscler. Thromb. 14, 47–53 (1994).
Bekkering, S. et al. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 34, 1731–1738 (2014).
Berliner, J. A. & Heinecke, J. W. The role of oxidized lipoproteins in atherogenesis. Free Radic. Biol. Med. 20, 707–727 (1996).
Berliner, J. A. et al. Oxidized lipids in atherogenesis: formation, destruction and action. Thromb. Haemost. 78, 195–199 (1997).
Holvoet, P. et al. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 21, 844–848 (2001).
Koenig, W. et al. Oxidized LDL and the risk of coronary heart disease: results from the MONICA/KORA Augsburg Study. Clin. Chem. 57, 1196–1200 (2011).
Murphy, A. J. et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 28, 2071–2077 (2008).
Barter, P. J. et al. Antiinflammatory properties of HDL. Circ. Res. 95, 764–772 (2004).
Wang, N., Ranalletta, M., Matsuura, F., Peng, F. & Tall, A. R. LXR-induced redistribution of ABCG1 to plasma membrane in macrophages enhances cholesterol mass efflux to HDL. Arterioscler. Thromb. Vasc. Biol. 26, 1310–1316 (2006).
Du, X.-M. et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ. Res. 116, 1133–1142 (2015).
Navab, M., Reddy, S. T., Van Lenten, B. J. & Fogelman, A. M. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 8, 222–232 (2011).
D'Agostino, R. B. et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117, 743–753 (2008).
Wilson, P. W., Abbott, R. D. & Castelli, W. P. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis 8, 737–741 (1988).
AIM-HIGH Investigators et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365, 2255–2267 (2011).
HPS2-THRIVE Collaborative Group et al. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 371, 203–212 (2014).
Barter, P. J. et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357, 2109–2122 (2007).
Schwartz, G. G. et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367, 2089–2099 (2012).
Nicholls, S. J. et al. Efficacy and safety of a novel oral inducer of apolipoprotein A-I synthesis in statin-treated patients with stable coronary artery disease. J. Am. Coll. Cardiol. 57, 1111–1119 (2011).
Nicholls, S. J. et al. ApoA-I induction as a potential cardioprotective strategy: rationale for the SUSTAIN and ASSURE studies. Cardiovasc. Drugs Ther. 26, 181–187 (2012).
Voight, B. F. et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 380, 572–580 (2012).
Mackey, R. H. et al. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J. Am. Coll. Cardiol. 60, 508–516 (2012).
Khera, A. V. et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364, 127–135 (2011).
Zilversmit, D. B. Atherogenesis: a postprandial phenomenon. Circulation 60, 473–485 (1979).
Proctor, S. D. & Mamo, J. C. Retention of fluorescent-labelled chylomicron remnants within the intima of the arterial wall — evidence that plaque cholesterol may be derived from post-prandial lipoproteins. Eur. J. Clin. Invest. 28, 497–503 (1998).
Ginsberg, H. N. et al. Association of postprandial triglyceride and retinyl palmitate responses with newly diagnosed exercise-induced myocardial ischemia in middle-aged men and women. Arterioscler. Thromb. Vasc. Biol. 15, 1829–1838 (1995).
Sharrett, A. R., Chambless, L. E., Heiss, G., Paton, C. C. & Patsch, W. Association of postprandial triglyceride and retinyl palmitate responses with asymptomatic carotid artery atherosclerosis in middle-aged men and women. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler. Thromb. Vasc. Biol. 15, 2122–2129 (1995).
Weintraub, M. S. et al. Clearance of chylomicron remnants in normolipidaemic patients with coronary artery disease: case control study over three years. BMJ 312, 935–939 (1996).
Karpe, F., Steiner, G., Uffelman, K., Olivecrona, T. & Hamsten, A. Postprandial lipoproteins and progression of coronary atherosclerosis. Atherosclerosis 106, 83–97 (1994).
Sarwar, N. et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 115, 450–458 (2007).
Shapiro, M. D. & Fazio, S. From lipids to inflammation: new approaches to reducing atherosclerotic risk. Circ. Res. 118, 732–749 (2016).
Thomsen, M., Varbo, A., Tybjærg-Hansen, A. & Nordestgaard, B. G. Low nonfasting triglycerides and reduced all-cause mortality: a Mendelian randomization study. Clin. Chem. 60, 737–746 (2014).
Jørgensen, A. B. et al. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur. Heart J. 34, 1826–1833 (2013).
Varbo, A., Benn, M., Tybjærg-Hansen, A. & Nordestgaard, B. G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 128, 1298–1309 (2013).
Johansen, C. T. et al. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat. Genet. 42, 684–687 (2010).
Do, R. et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 45, 1345–1352 (2013).
Wittrup, H. H., Tybjaerg-Hansen, A. & Nordestgaard, B. G. Lipoprotein lipase mutations, plasma lipids and lipoproteins, and risk of ischemic heart disease. A meta-analysis. Circulation 99, 2901–2907 (1999).
Wu, H. et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation 119, 2708–2717 (2009).
den Hartigh, L. J., Connolly-Rohrbach, J. E., Fore, S., Huser, T. R. & Rutledge, J. C. Fatty acids from very low-density lipoprotein lipolysis products induce lipid droplet accumulation in human monocytes. J. Immunol. 184, 3927–3936 (2010).
Gower, R. M. et al. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler. Thromb. Vasc. Biol. 31, 160–166 (2011).
Guijas, C. et al. Simultaneous activation of p38 and JNK by arachidonic acid stimulates the cytosolic phospholipase A2-dependent synthesis of lipid droplets in human monocytes. J. Lipid Res. 53, 2343–2354 (2012).
Foster, G. A., Gower, R. M. & Stanhope, K. L. On-chip phenotypic analysis of inflammatory monocytes in atherogenesis and myocardial infarction. Proc. Natl Acad. Sci. USA 110, 13944–13949 (2013).
Xu, L. et al. Foamy monocytes form early and contribute to nascent atherosclerosis in mice with hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 35, 1787–1797 (2015).
Khan, I. M. et al. Postprandial monocyte activation in individuals with metabolic syndrome. J. Clin. Endocrinol. Metab. 101, 4195–4204 (2016).
Foster, G. A. et al. CD11c/CD18 signals very late antigen-4 activation to initiate foamy monocyte recruitment during the onset of hypercholesterolemia. J. Immunol. 195, 5380–5392 (2015).
Delgado-Rodríguez, M., Medina-Cuadros, M., Martínez-Gallego, G. & Sillero-Arenas, M. Total cholesterol, HDL-cholesterol, and risk of nosocomial infection: a prospective study in surgical patients. Infect. Control Hosp. Epidemiol. 18, 9–18 (1997).
Netea, M. G. et al. Hyperlipoproteinemia enhances susceptibility to acute disseminated Candida albicans infection in low-density-lipoprotein-receptor-deficient mice. Infect. Immun. 65, 2663–2667 (1997).
de Bont, N. et al. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J. Lipid Res. 40, 680–685 (1999).
Roselaar, S. E. & Daugherty, A. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J. Lipid Res. 39, 1740–1743 (1998).
Suzuki, M. & O'Neal, R. M. Accumulation of lipids in the leukocytes of rats fed atherogenic diets. J. Lipid Res. 5, 624–627 (1964).
Gerrity, R. G., Naito, H. K., Richardson, M. & Schwartz, C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am. J. Pathol. 95, 775–792 (1979).
Gerrity, R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am. J. Pathol. 103, 181–190 (1981).
Choi, S.-H. et al. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ. Res. 104, 1355–1363 (2009).
Dresel, H. A. et al. Observations on leukocytes from patients with severe familial hypercholesterolemia. Arteriosclerosis 6, 259–264 (1986).
Mosig, S. et al. Different functions of monocyte subsets in familial hypercholesterolemia: potential function of CD14+ CD16+ monocytes in detoxification of oxidized LDL. FASEB J. 23, 866–874 (2009).
Steinberg, D. The LDL modification hypothesis of atherogenesis: an update. J. Lipid Res. 50 (Suppl.), S376–S381 (2009).
Fuhrman, B. et al. Atorvastatin therapy in hypercholesterolemic patients suppresses cellular uptake of oxidized-LDL by differentiating monocytes. Atherosclerosis 164, 179–185 (2002).
Li, L. et al. The importance of GLUT3 for de novo lipogenesis in hypoxia-induced lipid loading of human macrophages. PLoS ONE 7, e42360 (2012).
Ross, R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Rajamäki, K. et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE 5, e11765 (2010).
Quinn, M. T., Parthasarathy, S., Fong, L. G. & Steinberg, D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc. Natl Acad. Sci. USA 84, 2995–2998 (1987).
Rajavashisth, T. B. et al. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature 344, 254–257 (1990).
Snodgrass, R. G., Huang, S., Choi, I.-W., Rutledge, J. C. & Hwang, D. H. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J. Immunol. 191, 4337–4347 (2013).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 (2011).
Shalhoub, J., Falck-Hansen, M. A., Davies, A. H. & Monaco, C. Innate immunity and monocyte-macrophage activation in atherosclerosis. J. Inflamm. (Lond.) 8, 9 (2011).
Michelsen, K. S. et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl Acad. Sci. USA 101, 10679–10684 (2004).
Lundberg, A. M. et al. Toll-like receptor 3 and 4 signalling through the TRIF and TRAM adaptors in haematopoietic cells promotes atherosclerosis. Cardiovasc. Res. 99, 364–373 (2013).
Kanters, E. et al. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 112, 1176–1185 (2003).
Kanters, E. et al. Hematopoietic NF-kappaB1 deficiency results in small atherosclerotic lesions with an inflammatory phenotype. Blood 103, 934–940 (2004).
Gaidt, M. M. et al. Human monocytes engage an alternative inflammasome pathway. Immunity 44, 833–846 (2016).
Menu, P. et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2, e137 (2011).
Stewart, C. R. et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161 (2010).
Pasare, C. & Medzhitov, R. Toll-like receptors: linking innate and adaptive immunity. Adv. Exp. Med. Biol. 560, 11–18 (2005).
Barlic, J., Zhang, Y., Foley, J. F. & Murphy, P. M. Oxidized lipid-driven chemokine receptor switch, CCR2 to CX3CR1, mediates adhesion of human macrophages to coronary artery smooth muscle cells through a peroxisome proliferator-activated receptor gamma-dependent pathway. Circulation 114, 807–819 (2006).
Frostegård, J. et al. Oxidized low density lipoprotein induces differentiation and adhesion of human monocytes and the monocytic cell line U937. Proc. Natl Acad. Sci. USA 87, 904–908 (1990).
Hayden, J. M. et al. Induction of monocyte differentiation and foam cell formation in vitro by 7-ketocholesterol. J. Lipid Res. 43, 26–35 (2002).
Spann, N. J. et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151, 138–152 (2012).
Guijas, C., Meana, C., Astudillo, A. M., Balboa, M. A. & Balsinde, J. Foamy monocytes are enriched in cis-7- hexadecenoic fatty acid (16:1n-9), a possible biomarker for early detection of cardiovascular disease. Cell Chem. Biol. 23, 689–699 (2016).
Zhu, X. et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 51, 3196–3206 (2010).
Murphy, A. J. et al. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 1333–1341 (2011).
Yvan-Charvet, L. et al. Increased inflammatory gene expression in ABC transporter–deficient macrophages. Circulation 118, 1837–1847 (2008).
Iqbal, A. J. et al. Acute exposure to apolipoprotein A1 inhibits macrophage chemotaxis in vitro and monocyte recruitment in vivo. eLife 5, e15190 (2016).
Tall, A. R. & Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116 (2015).
Björkegren, J., Karpe, F., Milne, R. W. & Hamsten, A. Differences in apolipoprotein and lipid composition between human chylomicron remnants and very low density lipoproteins isolated from fasting and postprandial plasma. J. Lipid Res. 39, 1412–1420 (1998).
Brynes, A. E. et al. A randomised four-intervention crossover study investigating the effect of carbohydrates on daytime profiles of insulin, glucose, non-esterified fatty acids and triacylglycerols in middle-aged men. Br. J. Nutr. 89, 207–218 (2003).
Nordestgaard, B. G., Benn, M., Schnohr, P. & Tybjærg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298, 299–308 (2007).
Nordestgaard, B. G. et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points — a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 37, 1944–1958 (2016).
Oh, J. & Hegele, R. A. HIV-associated dyslipidaemia: pathogenesis and treatment. Lancet Infect. Dis. 7, 787–796 (2007).
Bruce, I. N. 'Not only...but also': factors that contribute to accelerated atherosclerosis and premature coronary heart disease in systemic lupus erythematosus. Rheumatology (Oxford) 44, 1492–1502 (2005).
Ishibashi, S., Herz, J., Maeda, N., Goldstein, J. L. & Brown, M. S. The two-receptor model of lipoprotein clearance: tests of the hypothesis in 'knockout' mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc. Natl Acad. Sci. USA 91, 4431–4435 (1994).
Cox, R. A. & García-Palmieri, M. R. in Clinical Methods: The History, Physical, and Laboratory Examinations Ch. 31 (eds Walker, H. K., Hall, W. D. & Hurst, J. W.) 153–160 (Butterworths, 1990).
Llodra, J. et al. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl Acad. Sci. USA 101, 11779–11784 (2004).
Trogan, E. et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl Acad. Sci. USA 103, 3781–3786 (2006).
Barcia, A. M. & Harris, H. W. Triglyceride-rich lipoproteins as agents of innate immunity. Clin. Infect. Dis. 41 (Suppl. 7), S498–S503 (2005).
Johnston, T. P. The P-407–induced murine model of dose-controlled hyperlipidemia and atherosclerosis: a review of findings to date. J. Cardiovasc. Pharmacol. 43, 595 (2004).
Ono-Moore, K. D. et al. Postprandial inflammatory responses and free fatty acids in plasma of adults who consumed a moderately high-fat breakfast with and without blueberry powder in a randomized placebo-controlled trial. J. Nutr. 146, 1411–1419 (2016).
Iqbal, R. et al. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation 118, 1929–1937 (2008).
Norris, J. N. Controlled trial of soya bean oil in myocardial infarction: report of a research committee to the Medical Research Council. Lancet 292, 693–700 (1968).
[No authors listed.] Los Angeles Veterans Administration diet study. Nutr. Rev. 27, 311–316 (1969).
Turpeinen, O. et al. Dietary prevention of coronary heart disease: the Finnish Mental Hospital Study. Int. J. Epidemiol. 8, 99–118 (1979).
Watts, G. F. et al. Effects on coronary artery disease of lipid-lowering diet, or diet plus cholestyramine, in the St Thomas' Atherosclerosis Regression Study (STARS). Lancet 339, 563–569 (1992).
Mozaffarian, D., Micha, R. & Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 7, e1000252 (2010).
Leren, P. The effect of plasma cholesterol lowering diet in male survivors of myocardial infarction. A controlled clinical trial. Acta Med. Scand. Suppl. 466, 1–92 (1966).
Brunner, E. J. et al. Dietary patterns and 15-y risks of major coronary events, diabetes, and mortality. Am. J. Clin. Nutr. 87, 1414–1421 (2008).
Schwingshackl, L. & Hoffmann, G. Comparison of effects of long-term low-fat versus high-fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta-analysis. J. Acad. Nutr. Diet. 113, 1640–1661 (2013).
Leren, P. The Oslo Diet-Heart Study: eleven-year report. Circulation 42, 935–942 (1970).
Khymenets, O. et al. Mononuclear cell transcriptome response after sustained virgin olive oil consumption in humans: an exploratory nutrigenomics study. OMICS 13, 7–19 (2009).
Estruch, R. et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 368, 1279–1290 (2013).
Bouwens, M. et al. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am. J. Clin. Nutr. 90, 415–424 (2009).
Bouwens, M., Grootte Bromhaar, M., Jansen, J., Müller, M. & Afman, L. A. Postprandial dietary lipid-specific effects on human peripheral blood mononuclear cell gene expression profiles. Am. J. Clin. Nutr. 91, 208–217 (2010).
Chowdhury, R. et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann. Intern. Med. 160, 398–406 (2014).
Eckel, R. H. et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 63, 2960–2984 (2014).
Harcombe, Z. et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Heart 2, e000196 (2015).
de Souza, R. J. et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 351, h3978 (2015).
Bellido, C. et al. Butter and walnuts, but not olive oil, elicit postprandial activation of nuclear transcription factor κB in peripheral blood mononuclear cells from healthy men. Am. J. Clin. Nutr. 80, 1487–1491 (2004).
Erridge, C., Attina, T., Spickett, C. M. & Webb, D. J. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 86, 1286–1292 (2007).
Amar, J. et al. Energy intake is associated with endotoxemia in apparently healthy men. Am. J. Clin. Nutr. 87, 1219–1223 (2008).
Ghoshal, S., Witta, J., Zhong, J., de Villiers, W. & Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 50, 90–97 (2008).
Ghanim, H. et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care 32, 2281–2287 (2009).
Cullberg, K. B., Larsen, J. O., Pedersen, S. B. & Richelsen, B. Effects of LPS and dietary free fatty acids on MCP-1 in 3T3-L1 adipocytes and macrophages in vitro. Nutr. Diabetes 4, e113 (2014).
Huang, S. et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 53, 2002–2013 (2012).
Eguchi, K. et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 15, 518–533 (2012).
Chait, A. & Kim, F. Saturated fatty acids and inflammation: who pays the toll? Arterioscler. Thromb. Vasc. Biol. 30, 692–693 (2010).
Youssef-Elabd, E. M. et al. Acute and chronic saturated fatty acid treatment as a key instigator of the TLR-mediated inflammatory response in human adipose tissue. in vitro. J. Nutr. Biochem. 23, 39–50 (2012).
Erridge, C. & Samani, N. J. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler. Thromb. Vasc. Biol. 29, 1944–1949 (2009).
Klop, B. et al. Leukocyte cell population data (volume conductivity scatter) in postprandial leukocyte activation. Int. J. Lab. Hematol. 35, 644–651 (2013).
Varela, L. M. et al. The effects of dietary fatty acids on the postprandial triglyceride-rich lipoprotein/apoB48 receptor axis in human monocyte/macrophage cells. J. Nutr. Biochem. 24, 2031–2039 (2013).
Bays, H. E. Adiposopathy is 'sick fat' a cardiovascular disease? J. Am. Coll. Cardiol. 57, 2461–2473 (2011).
Bays, H. E. et al. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. J. Clin. Lipidol. 7, 304–383 (2013).
Bays, H. E. Adiposopathy, diabetes mellitus, and primary prevention of atherosclerotic coronary artery disease: treating 'sick fat' through improving fat function with antidiabetes therapies. Am. J. Cardiol. 110, 4B–12B (2012).
Pearce, E. N. Update in lipid alterations in subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 97, 326–333 (2012).
Omran, J., Al-Dadah, A. & Dellsperger, K. C. Dyslipidemia in patients with chronic and end-stage kidney disease. Cardiorenal Med. 3, 165–177 (2013).
Kolovou, G. D., Anagnostopoulou, K. K., Kostakou, P. M., Bilianou, H. & Mikhailidis, D. P. Primary and secondary hypertriglyceridaemia. Curr. Drug Targets 10, 336–343 (2009).
Kasiske, B. L. Effects of antihypertensive therapy on serum lipids. Ann. Intern. Med. 122, 133–141 (1995).
Darling, G. M., Johns, J. A., McCloud, P. I. & Davis, S. R. Estrogen and progestin compared with simvastatin for hypercholesterolemia in postmenopausal women. N. Engl. J. Med. 337, 595–601 (1997).
Lobo, R. A., Skinner, J. B., Lippman, J. S. & Cirillo, S. J. Plasma lipids and desogestrel and ethinyl estradiol: a meta-analysis. Fertil. Steril. 65, 1100–1109 (1996).
Henderson, D. C. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am. J. Psychiatry 157, 975–981 (2000).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Serbina, N. V. & Pamer, E. G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 (2006).
Debien, E. et al. S1PR5 is pivotal for the homeostasis of patrolling monocytes. Eur. J. Immunol. 43, 1667–1675 (2013).
Tacke, F. Functional role of intrahepatic monocyte subsets for the progression of liver inflammation and liver fibrosis in vivo. Fibrogenesis Tissue Repair 5, S27 (2012).
Acknowledgements
K.J.W.'s laboratory is funded by BHF CRTF to M.S.R. (FS/14/50/30856), MRC (MR/M003159/1), Kidney Research UK (RP_019_20160303), and Imperial Biomedical Research Centre. A.J.M is supported by a Career Development Fellowship from the NHMRC, a Future Leader Fellowship from the NHF, and Project Grants from the NHMRC and Diabetes Australia. We thank members of the Woollard lab for helpful discussion and lab work underpinning data for this Review.
Author information
Authors and Affiliations
Contributions
M.S.R. and K.J.W. researched data for the article and wrote the manuscript. A.J.M. and K.J.W. provided substantial contribution to the discussion of content, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Mononuclear phagocyte system
-
Part of the immune system that consists of phagocytic cells (monocytes and macrophages), but also tissue-specific phagocytes such as Kupffer cells, microglia, osteoclasts, and Langerhans cells.
- Adoptive transfer
-
A technique for introducing labelled cells of interest into a host animal for the purposes of tracking and/or imaging, or to ascertain effects of the donor cells on a host.
- Parabiosis models
-
Used to study the effects of one biological system on another through the surgical conjoining of circulatory and physiological systems. Mice and rats are commonly used in these models.
- Aorta–gonad–mesonephros
-
Region of the embryonic mesoderm that includes the dorsal aorta and is implicated in definitive haematopoietic stem cell myelopoiesis.
- Common monocyte progenitor
-
Monocyte progenitor cell in the bone marrow distinct from macrophage and dendritic cell precursor cells.
- Plasmacytoid dendritic cells
-
Although their appearance resembles that of plasma cells, the function of these cells resembles that of conventional myeloid dendritic cells.
- Lipoprotein
-
A carrier molecule that contains and transports lipid (cholesterol and triglyceride) in the circulation, and consists of apolipoproteins and a single-layer phospholipid membrane. Examples include HDL, LDL, VLDL, and chylomicrons.
- ATP-binding cassette transporters
-
Actively transport molecules into or out of cells. An example is the ABCA1 transporter that has a role in cholesterol export to HDL.
- Oxidized LDL
-
LDL molecule in which the lipoprotein envelope and/or cholesterol content has been oxidized by free radicals. Oxidized forms of LDL are thought to have an important role in atherosclerosis, and can stimulate macrophages to accumulate lipid and accelerate conversion to foam cells.
- Reverse cholesterol transport
-
Transport of cholesterol from tissues or cells back to the liver for clearance. Cholesterol can be transported via ABCA1 to HDL, which acts as an acceptor molecule. This process is thought to be cardioprotective.
- Neutral lipid droplets
-
Lipids without a polar charge that can be found within cells, and commonly refers to cholesteryl esters and triglycerides.
- NLRP3 inflammasome
-
An important oligomer molecule that forms part of the cellular inflammatory response, particularly in innate immune cells. Its activation results in the release of cytokines such as interleukin-1β.
- Minimally modified LDL
-
A unique form of oxidized LDL enriched with aldehyde-containing phosphatidylcholines.
- Reconstituted HDL
-
A synthetic form of HDL used to improve cholesterol transport out of the cell (efflux) with potential therapeutic value in cardiovascular disease.
- Chylomicrons
-
Also known as 'ultra-low density lipoprotein', these large lipoprotein particles are secreted by gut endothelial cells and predominantly transport dietary lipids (triglycerides and cholesterol) in the circulation. Chylomicron levels rise after dietary intake of fat or carbohydrate and fall after fasting.
- Very low-density lipoprotein
-
These lipoproteins predominantly transport triglycerides produced by the body (muscle, liver), but can also be altered by dietary fat intake.
Rights and permissions
About this article
Cite this article
Rahman, M., Murphy, A. & Woollard, K. Effects of dyslipidaemia on monocyte production and function in cardiovascular disease. Nat Rev Cardiol 14, 387–400 (2017). https://doi.org/10.1038/nrcardio.2017.34
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2017.34
This article is cited by
-
Dysregulated cellular metabolism in atherosclerosis: mediators and therapeutic opportunities
Nature Metabolism (2024)
-
Monocyte-to-high-density lipoprotein-cholesterol ratio (MHR) and the risk of all-cause and cardiovascular mortality: a nationwide cohort study in the United States
Lipids in Health and Disease (2022)
-
Chronic kidney disease mediates cardiac dysfunction associated with increased resident cardiac macrophages
BMC Nephrology (2022)
-
Radial BMD and serum CTX-I can predict the progression of carotid plaque in rheumatoid arthritis: a 3-year prospective cohort study
Arthritis Research & Therapy (2021)
-
Lipid metabolism, inflammation, and foam cell formation in health and metabolic disorders: targeting mTORC1
Journal of Molecular Medicine (2021)