Key Points
-
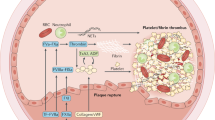
The non-vitamin K antagonist oral anticoagulants (NOACs) include dabigatran, which inhibits thrombin, and apixaban, betrixaban, edoxaban, and rivaroxaban, which inhibit factor Xa
-
Three NOAC reversal agents are in various stages of development: idarucizumab is licensed in many countries, andexanet is under consideration by regulatory agencies, and ciraparantag is undergoing phase III evaluation
-
Idarucizumab is approved for dabigatran reversal in patients requiring emergency surgery or urgent procedures and in those with life-threatening or uncontrolled bleeding
-
In the absence of licensed reversal agents for the oral factor Xa inhibitors, prothrombin complex concentrates are increasingly used in patients taking these agents who present with life-threatening bleeding
Abstract
The non-vitamin K antagonist oral anticoagulants (NOACs) include dabigatran, which inhibits thrombin, and apixaban, betrixaban, edoxaban, and rivaroxaban, which inhibit coagulation factor Xa. Although clinical studies of NOACs were conducted without antidotes, patient outcomes with major bleeding when receiving NOACs were no worse than those in patients treated with a vitamin K antagonist. Nonetheless, in patients with life-threatening bleeding or requiring urgent surgery, the capacity for rapid NOAC reversal is likely to increase patient safety. Three NOAC reversal agents are in various stages of development: idarucizumab, a specific reversal agent for dabigatran; andexanet alfa, which reverses factor Xa inhibitors; and ciraparantag, which is purported to reverse all NOACs. Idarucizumab is licensed in many countries, andexanet is under consideration by regulatory agencies, and ciraparantag is undergoing phase III evaluation. In the absence of licensed reversal agents for the oral factor Xa inhibitors, prothrombin complex concentrates are often used in patients taking these agents who present with life-threatening bleeding. In this Review, we summarize the approved indications for the NOACs, outline how to measure their anticoagulant effects, describe the mechanism of action of the reversal strategies, assess the preclinical and clinical data supporting their use, provide guidance on potential indications for reversal, and offer a management approach for patients treated with NOACs who present with serious bleeding or require urgent surgery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Yeh, C. H., Gross, P. L. & Weitz, J. I. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood 124, 1020–1028 (2014).
Ruff, C. T. et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 383, 955–962 (2014).
Levy, J. H., Spyropoulos, A. C., Samama, C. M. & Douketis, J. Direct oral anticoagulants: new drugs and new concepts. JACC Cardiovasc. Interv. 7, 1333–1351 (2014).
Chan, N. C., Eikelboom, J. W. & Weitz, J. I. Evolving treatments for arterial and venous thrombosis: role of the direct oral anticoagulants. Circ. Res. 118, 1409–1424 (2016).
Sherwood, M. W. et al. Gastrointestinal bleeding in patients with atrial tibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J. Am. Coll. Cardiol. 66, 2271–2281 (2015).
Myers, B. & Webster, A. Heavy menstrual bleeding on rivaroxaban — comparison with apixaban. Br. J. Haematol. 176, 833–835 (2017).
Weitz, J. I. & Harenberg, J. New developments in anticoagulants: past, present and future. Thromb. Haemost. 117, 1283–1288 (2017).
Levy, J. H. et al. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J. Thromb. Haemost. 14, 623–627 (2016).
Cohen, A. T., Harrington, R. A. & Gibson, C. M. Betrixaban in acutely ill medical patients. N. Engl. J. Med. 375, e50 (2016).
Reiffel, J. A. et al. NOAC monitoring, reversal agents, and post-approval safety and effectiveness evaluation: a cardiac safety research consortium think tank. Am. Heart J. 177, 74–86 (2016).
Samuelson, B. T. & Cuker, A. Measurement and reversal of the direct oral anticoagulants. Blood Rev. 31, 77–84 (2017).
Ebner, M. et al. Emergency coagulation assessment during treatment with direct oral anticoagulants: limitations and solutions. Stroke 48, 2457–2463 (2017).
Dale, B. J., Chan, N. C. & Eikelboom, J. W. Laboratory measurement of the direct oral anticoagulants. Br. J. Haematol. 172, 315–336 (2016).
Gouin-Thibault, I. et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb. Haemost. 111, 240–248 (2014).
Cuker, A., Siegal, D. M., Crowther, M. A. & Garcia, D. A. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J. Am. Coll. Cardiol. 64, 1128–1139 (2014).
Konigsbrugge, O. et al. Anti-coagulation assessment with prothrombin time and anti-Xa assays in real-world patients on treatment with rivaroxaban. Ann. Hematol. 94, 1463–1471 (2015).
Pollack, C. V. Jr et al. Idarucizumab for dabigatran reversal — full cohort analysis. N. Engl. J. Med. 377, 431–441 (2017).
van Ryn, J. et al. Dabigatran etexilate — a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb. Haemost. 103, 1116–1127 (2010).
Jaffer, I. H. et al. Comparison of the ecarin chromogenic assay and diluted thrombin time for quantification of dabigatran concentrations. J. Thromb. Haemost. 15, 2377–2387 (2017).
Cuker, A. Laboratory measurement of the non-vitamin K antagonist oral anticoagulants: selecting the optimal assay based on drug, assay availability, and clinical indication. J. Thromb. Thrombolysis 41, 241–247 (2016).
Ikeda, K. & Tachibana, H. Clinical implication of monitoring rivaroxaban and apixaban by using anti-factor Xa assay in patients with non-valvular atrial fibrillation. J. Arrhythm. 32, 42–50 (2016).
Glund, S. et al. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb. Haemost. 113, 943–951 (2015).
Glund, S. et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet 386, 680–690 (2015).
Glund, S. et al. Effect of age and renal function on idarucizumab pharmacokinetics and idarucizumab-mediated reversal of dabigatran anticoagulant activity in a randomized, double-blind, crossover phase Ib study. Clin. Pharmacokinet. 56, 41–54 (2017).
Pollack, C. V. Jr et al. Idarucizumab for dabigatran reversal. N. Engl. J. Med. 373, 511–520 (2015).
Pollack, C. V. Jr et al. Design and rationale for RE-VERSE AD: a phase 3 study of idarucizumab, a specific reversal agent for dabigatran. Thromb. Haemost. 114, 198–205 (2015).
Crowther, M. & Crowther, M. A. Antidotes for novel oral anticoagulants: current status and future potential. Arterioscler. Thromb. Vasc. Biol. 35, 1736–1745 (2015).
Ghadimi, K., Dombrowski, K. E., Levy, J. H. & Welsby, I. J. Andexanet alfa for the reversal of factor Xa inhibitor related anticoagulation. Expert Rev. Hematol. 9, 115–122 (2016).
Siegal, D. M. et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N. Engl. J. Med. 373, 2413–2424 (2015).
Crowther, M. et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of edoxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (fXa) inhibitors [abstract]. Blood 124, 4269 (2014).
Crowther, M. et al. Reversal of betrixaban-induced anticoagulation in healthy volunteers by andexanet alfa [abstract]. Blood 128, 143 (2016).
Lu, G. et al. Preclinical safety and efficacy of andexanet alfa in animal models. J. Thromb. Haemost. 15, 1747–1756 (2017).
Connolly, S. J. et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N. Engl. J. Med. 375, 1131–1141 (2016).
Sullivan, D. W. Jr, Gad, S. C., Laulicht, B., Bakhru, S. & Steiner, S. Nonclinical safety assessment of PER977: a small molecule reversal agent for new oral anticoagulants and heparins. Int. J. Toxicol. 34, 308–317 (2015).
Ansell, J. E. et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N. Engl. J. Med. 371, 2141–2142 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03172910 (2017).
Eerenberg, E. S. et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 124, 1573–1579 (2011).
Levy, J. H. & Levi, M. New oral anticoagulant-induced bleeding: clinical presentation and management. Clin. Lab. Med. 34, 575–586 (2014).
Song, Y. et al. Reversal of apixaban anticoagulation by 4-factor prothrombin complex concentrates in healthy subjects: a randomized 3-period crossover study. J. Thromb. Haemost. 15, 2125–2137 (2017).
Zahir, H. et al. Edoxaban effects on bleeding following punch biopsy and reversal by a 4-factor prothrombin complex concentrate. Circulation 131, 82–90 (2015).
Levy, J. H. et al. Rivaroxaban reversal with prothrombin complex concentrate or tranexamic acid in healthy volunteers. J. Thromb. Haemost. http://dx.doi.org/10.1111/jth.13894 (2017).
Majeed, A. et al. Management of rivaroxaban or apixaban associated major bleeding with prothrombin complex concentrates: a cohort study. Blood 130, 1706–1712 (2017).
Varadi, K. et al. Pro- and anticoagulant factors facilitate thrombin generation and balance the haemostatic response to FEIBA® in prophylactic therapy. Haemophilia 22, 615–624 (2016).
Goodnough, L. T. & Levy, J. H. The judicious use of recombinant factor VIIa. Semin. Thromb. Hemost. 42, 125–132 (2016).
Marlu, R. et al. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. A randomised crossover ex vivo study in healthy volunteers. Thromb. Haemost. 108, 217–224 (2012).
Perzborn, E., Heitmeier, S., Laux, V. & Buchmuller, A. Reversal of rivaroxaban-induced anticoagulation with prothrombin complex concentrate, activated prothrombin complex concentrate and recombinant activated factor VII in vitro. Thromb. Res. 133, 671–681 (2014).
Fukuda, T., Honda, Y., Kamisato, C., Morishima, Y. & Shibano, T. Reversal of anticoagulant effects of edoxaban, an oral, direct factor Xa inhibitor, with haemostatic agents. Thromb. Haemost. 107, 253–259 (2012).
Halim, A. B., Samama, M. M. & Mendell, J. Ex vivo reversal of the anticoagulant effects of edoxaban. Thromb. Res. 134, 909–913 (2014).
Dibu, J. R., Weimer, J. M., Ahrens, C., Manno, E. & Frontera, J. A. The role of FEIBA in reversing novel oral anticoagulants in intracerebral hemorrhage. Neurocrit. Care 24, 413–419 (2016).
Mao, G., King, L., Young, S. & Kaplan, R. Factor eight inhibitor bypassing agent (FEIBA) for reversal of target-specific oral anticoagulants in life-threatening intracranial bleeding. J. Emerg. Med. 52, 731–737 (2017).
Mayer, S. A. et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N. Engl. J. Med. 358, 2127–2137 (2008).
Mayer, S. A. et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N. Engl. J. Med. 352, 777–785 (2005).
Tanaka, K. A., Szlam, F., Dickneite, G. & Levy, J. H. Effects of prothrombin complex concentrate and recombinant activated factor VII on vitamin K antagonist induced anticoagulation. Thromb. Res. 122, 117–123 (2008).
Levi, M., Levy, J. H., Andersen, H. F. & Truloff, D. Safety of recombinant activated factor VII in randomized clinical trials. N. Engl. J. Med. 363, 1791–1800 (2010).
Wang, X. et al. Effect of activated charcoal on apixaban pharmacokinetics in healthy subjects. Am. J. Cardiovasc. Drugs 14, 147–154 (2014).
Ollier, E. et al. Effect of activated charcoal on rivaroxaban complex absorption. Clin. Pharmacokinet. 56, 793–801 (2017).
Raval, A. N. et al. Management of patients on non-vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting: a Scientific Statement from the American Heart Association. Circulation 135, e604–e633 (2017).
Levy, J. H. et al. Antifibrinolytic therapy and perioperative considerations. Anesthesiology http://dx.doi.org/10.1097/ALN.0000000000001997 (2017).
Levy, J. H. Antifibrinolytic therapy: new data and new concepts. Lancet 376, 3–4 (2010).
Witt, D. M. What to do after the bleed: resuming anticoagulation after major bleeding. Hematol. Am. Soc. Hematol. Educ. Program 2016, 620–624 (2016).
Kuramatsu, J. B. et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 313, 824–836 (2015).
Nielsen, P. B., Larsen, T. B., Skjoth, F. &, Lip, G. Y. Outcomes associated with resuming warfarin treatment after hemorrhagic stroke or traumatic intracranial hemorrhage in patients with atrial fibrillation. JAMA Intern. Med. 177, 563–570 (2017).
Majeed, A. et al. Optimal timing of vitamin K antagonist resumption after upper gastrointestinal bleeding: a risk modelling analysis. Thromb. Haemost 117, 491–499 (2017).
Eikelboom, J. W. & Weitz, J. I. New anticoagulants. Circulation 121, 1523–1532 (2010).
Heidbuchel, H. et al. Updated European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 17, 1467–1507 (2015).
Seyve, L., Richarme, C., Polack, B. & Marlu, R. Impact of four direct oral anticoagulants on rotational thromboelastometry (ROTEM). Int. J. Lab. Hematol. http://dx.doi.org/10.1111/ijlh.12744 (2017).
Taune, V. et al. Whole blood coagulation assays ROTEM and T-TAS to monitor dabigatran treatment. Thromb. Res. 153, 76–82 (2017).
Ansell, J. E. et al. Single-dose ciraparantag safely and completely reverses anticoagulant effects of edoxaban. Thromb. Haemost. 117, 238–245 (2017).
Author information
Authors and Affiliations
Contributions
J.H.L. researched data for the article, and all the authors discussed its content. J.H.L. and J.I.W. wrote the manuscript, and all the authors reviewed and edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
J.H.L. is on the scientific advisory or steering committees of Boehringer Ingelheim, CSL Behring, Grifols, Instrumentation Laboratories, Janssen, Leading Biosciences, Merck, Octapharma, Pfizer, and Portola. J.D. is on the scientific advisory board of AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Leo Pharma, Pfizer, and Sanofi; and is a consultant for Janssen. J.I.W. served as a consultant and received honoraria from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Janssen, Novartis, and Pfizer.
Rights and permissions
About this article
Cite this article
Levy, J., Douketis, J. & Weitz, J. Reversal agents for non-vitamin K antagonist oral anticoagulants. Nat Rev Cardiol 15, 273–281 (2018). https://doi.org/10.1038/nrcardio.2017.223
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2017.223
This article is cited by
-
Effect of antiplatelet and anticoagulant medication use on injury severity and mortality in patients with traumatic brain injury treated in the intensive care unit
Acta Neurochirurgica (2023)
-
Bleeding disorders in implant dentistry: a narrative review and a treatment guide
International Journal of Implant Dentistry (2022)
-
Perioperative management of antithrombotic therapy: a case-based narrative review
Internal and Emergency Medicine (2022)
-
Do Oral Factor Xa Inhibitors have a Role in Patients with Mechanical Heart Valves?
American Journal of Cardiovascular Drugs (2022)
-
Dental implants and risk of bleeding in patients on oral anticoagulants: a systematic review and meta-analysis
International Journal of Implant Dentistry (2021)