Key Points
-
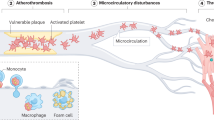
Antiplatelet agents form a cornerstone of therapy for patients with acute coronary syndrome undergoing percutaneous coronary intervention, as well as in the secondary prevention of cardiovascular events
-
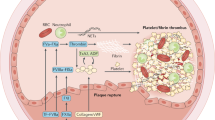
Currently available antiplatelet agents, including cyclooxygenase 1 inhibitors, P2Y purinoreceptor 12 (P2Y12) antagonists, protease-activated receptor 1 antagonists, and glycoprotein (GP) IIb/IIIa antagonists, inhibit processes important for both thrombosis and haemostasis
-
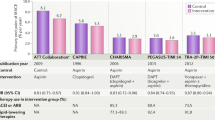
Bleeding remains a major limitation of current therapeutic approaches, with the most intensive antithrombotic regimens associated with an increased risk of bleeding
-
The adverse effects of bleeding on mortality and cardiovascular outcomes might offset the benefit of potent antiplatelet strategies
-
Experimental work has highlighted that thrombus formation in vivo is a dynamic process; new regulators of thrombus formation and, therefore, therapeutic targets that do not impair haemostasis have been identified
-
New antiplatelet strategies, including inhibitors of phosphatidylinositol 3-kinase-β (PI3Kβ), protein disulfide-isomerase (PDI), activated GPIIb/IIIa, GPIIb/IIIa outside-in signalling, protease-activated receptors, and platelet GPVI-mediated adhesion pathways, are in preclinical and early-phase clinical trials
Abstract
Antiplatelet drugs, such as aspirin, P2Y12 antagonists, and glycoprotein (GP) IIb/IIIa inhibitors, have proved to be successful in reducing the morbidity and mortality associated with arterial thrombosis. These agents are, therefore, the cornerstone of therapy for patients with acute coronary syndromes. However, these drugs all carry an inherent risk of bleeding, which is associated with adverse cardiovascular outcomes and mortality. Thus, the potential benefits of more potent, conventional antiplatelet drugs are likely be offset by the increased risk of bleeding. Data from experiments in vivo have highlighted potentially important differences between haemostasis and thrombosis, raising the prospect of developing new antiplatelet drugs that are not associated with bleeding. Indeed, in preclinical studies, several novel antiplatelet therapies that seem to inhibit thrombosis while maintaining haemostasis have been identified. These agents include inhibitors of phosphatidylinositol 3-kinase-β (PI3Kβ), protein disulfide-isomerase, activated GPIIb/IIIa, GPIIb/IIIa outside-in signalling, protease-activated receptors, and platelet GPVI-mediated adhesion pathways. In this Review, we discuss how a therapeutic ceiling has been reached with existing antiplatelet drugs, whereby increased potency is offset by elevated bleeding risk. The latest advances in our understanding of thrombus formation have informed the development of new antiplatelet drugs that are potentially safer than currently available therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
16 January 2018
In the version of this article initially published online, joint first authorship was not attributed to James D. McFadyen and Mathieu Schaff. This error has been corrected for the HTML, print and PDF versions of the article.
References
Naghavi, M. et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385, 117–171 (2015).
Mendis, S., Puska, P. & Norrving, B. in Global Atlas on Cardiovascular Disease Prevention and Control (eds Mendis, S., Puska, P. & Norrving, B) 113 (WHO, 2011).
Bloom, D. E. et al. The Global Economic Burden of Non-communicable Diseases (World Economic Forum, 2011).
Falk, E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br. Heart J. 50, 127–134 (1983).
Ruggeri, Z. M. Structure and function of von Willebrand factor. Thromb. Haemost. 82, 576–584 (1999).
Ruggeri, Z. M. Platelet adhesion under flow. Microcirculation 16, 58–83 (2009).
McFadyen, J. D. & Jackson, S. P. Differentiating haemostasis from thrombosis for therapeutic benefit. Thromb. Haemost. 110, 859–867 (2013).
Dütting, S., Bender, M. & Nieswandt, B. Platelet GPVI: a target for antithrombotic therapy?! Trends Pharmacol. Sci. 11, 583–590 (2012).
Jin, J., Daniel, J. L. & Kunapuli, S. P. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J. Biol. Chem. 273, 2030–2034 (1998).
Huang, J. S., Ramamurthy, S. K., Lin, X. & Le Breton, G. C. Cell signalling through thromboxane A2 receptors. Cell Signal. 16 521–533 (2004).
Coughlin, S. R. How the protease thrombin talks to cells. Proc. Natl Acad. Sci. USA 96, 11023–11027 (1999).
Bennett, J. S. Structure and function of the platelet integrin αIIbβ3 . J. Clin. Invest. 115, 3363–3369 (2005).
Nieswandt, B., Varga-Szabo, D. & Elvers, M. Integrins in platelet activation. J. Thromb. Haemost. 7 (Suppl. 1), 206–209 (2009).
Furie, B. & Furie, B. C. Mechanisms of thrombus formation. N. Engl. J. Med. 359, 938–949 (2008).
Ishii, K., Hein, L., Kobilka, B. & Coughlin, S. R. Kinetics of thrombin receptor cleavage on intact cells. Relation to signaling. J. Biol. Chem. 268, 9780–9786 (1993).
Covic, L., Singh, C., Smith, H. & Kuliopulos, A. Role of the PAR4 thrombin receptor in stabilizing platelet-platelet aggregates as revealed by a patient with Hermansky-Pudlak syndrome. Thromb. Haemost. 87, 722–727 (2002).
Nesbitt, W. S. et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat. Med. 15, 665–673 (2009).
Stalker, T. J. et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood 121, 1875–1885 (2013).
Welsh, J. D. et al. A systems approach to hemostasis: 1. The interdependence of thrombus architecture and agonist movements in the gaps between platelets. Blood 124, 1808–1815 (2014).
Stalker, T. J. et al. A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood 124, 1824–1831 (2014).
Jackson, S. P. et al. PI 3-kinase p110β: a new target for antithrombotic therapy. Nat. Med. 11, 507–514 (2005).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874 (2002).
Li, Z., Delaney, M. K., O'Brien, K. A. & Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 30, 2341–2349 (2010).
Shattil, S. J. & Newman, P. J. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood 104, 1606–1615 (2004).
Shen, B. et al. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature 503, 131–135 (2013).
Furie, B. & Flaumenhaft, R. Thiol isomerases in thrombus formation. Circ. Res. 114, 1162–1173 (2014).
Cho, J., Furie, B. C., Coughlin, S. R. & Furie, B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J. Clin. Invest. 118, 1123–1131 (2008).
Kim, K. et al. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood 122, 1052–1061 (2013).
Yousuf, O. & Bhatt, D. L. The evolution of antiplatelet therapy in cardiovascular disease. Nat. Rev. Cardiol. 8, 547–559 (2011).
Deo, S. V. et al. Dual anti-platelet therapy after coronary artery bypass grafting: is there any benefit? A systematic review and meta-analysis. J. Card. Surg. 28, 109–116 (2013).
Ferreiro, J. L. & Angiolillo, D. J. New directions in antiplatelet therapy. Circ. Cardiovasc. Interv. 5, 433–445 (2012).
Hennekens, C. H., Dyken, M. L. & Fuster, V. Aspirin as a therapeutic agent in cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 96, 2751–2753 (1997).
Lewis, H. D. Jr et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina — results of a Veterans Administration cooperative study. N. Engl. J. Med. 309, 396–403 (1983).
ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 2, 349–360 (1988).
Antithrombotic Trialists Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324, 71–86 (2002).
Wallentin, L. P2Y(12) inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. Eur. Heart J. 30, 1964–1977 (2009).
Yusuf, S. et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 345, 494–502 (2001).
Erlinge, D. et al. Patients with poor responsiveness to thienopyridine treatment or with diabetes have lower levels of circulating active metabolite, but their platelets respond normally to active metabolite added ex vivo. J. Am. Coll. Cardiol. 52, 1968–1977 (2008).
Wallentin, L. et al. Prasugrel achieves greater and faster P2Y12 receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur. Heart J. 29, 21–30 (2008).
Wallentin, L. et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361, 1045–1057 (2009).
Husted, S. et al. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur. Heart J. 27, 1038–1047 (2006).
Franchi, F., Rollini, F., Muniz-Lozano, A., Cho, J. R. & Angiolillo, D. J. Cangrelor: a review on pharmacology and clinical trial development. Expert Rev. Cardiovasc. Ther. 11, 1279–1291 (2013).
Bhatt, D. L. et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N. Engl. J. Med. 368, 1303–1313 (2013).
Coughlin, S. R. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 3, 1800–1814 (2005).
Kalz, J., ten Cate, H. & Spronk, H. M. Thrombin generation and atherosclerosis. J. Thromb. Thrombolysis 37, 45–55 (2014).
Bonaca, M. P. et al. Antithrombotics in acute coronary syndromes. J. Am. Coll. Cardiol. 54, 969–984 (2009).
Morrow, D. A. et al. Vorapaxar in the secondary prevention of atherothrombotic events. N. Engl. J. Med. 366, 1404–1413 (2012).
Franchi, F. & Angiolillo, D. J. Novel antiplatelet agents in acute coronary syndrome. Nat. Rev. Cardiol. 12, 30–47 (2015).
Tricoci, P. et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N. Engl. J. Med. 366, 20–33 (2012).
Michelson, A. D. Antiplatelet therapies for the treatment of cardiovascular disease. Nat. Rev. Drug Discov. 9, 154–169 (2010).
Bhatt, D. L. & Topol, E. J. Current role of platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes. JAMA 284, 1549–1558 (2000).
Kastrati, A. et al. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 295, 1531–1538 (2006).
Bosch, X., Marrugat, J. & Sanchis, J. Platelet glycoprotein IIb/IIIa blockers during percutaneous coronary intervention and as the initial medical treatment of non-ST segment elevation acute coronary syndromes. Cochrane Database Syst. Rev. 11, CD002130 (2013).
Serebruany, V. L., Malinin, A. I., Eisert, R. M. & Sane, D. C. Risk of bleeding complications with antiplatelet agents: meta-analysis of 338,191 patients enrolled in 50 randomized controlled trials. Am. J. Hematol. 75, 40–47 (2004).
Wiviott, S. D. et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357, 2001–2015 (2007).
Levine, G. N. et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 134, e123–e155 (2016).
Windecker, S. et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS): developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 35, 2541–2619 (2014).
Mehran, R. et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 123, 2736–2747 (2011).
Mehta, S. R. et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet 376, 1233–1243 (2010).
Rao, S. V. et al. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur. Heart J. 28, 1193–1204 (2007).
Eikelboom, J. W. et al. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 114, 774–782 (2006).
Manoukian, S. V. et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J. Am. Coll. Cardiol. 49, 1362–1368 (2007).
Doyle, B. J., Rihal, C. S., Gastineau, D. A. & Holmes, D. R. Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J. Am. Coll. Cardiol. 53, 2019–2027 (2009).
Ducrocq, G. et al. Association of spontaneous and procedure-related bleeds with short- and long-term mortality after acute coronary syndromes: an analysis from the PLATO trial. EuroIntervention 11, 737–745 (2015).
Silvain, J. et al. Impact of red blood cell transfusion on platelet aggregation and inflammatory response in anemic coronary and noncoronary patients: the TRANSFUSION-2 study (impact of transfusion of red blood cell on platelet activation and aggregation studied with flow cytometry use and light transmission aggregometry). J. Am. Coll. Cardiol. 63, 1289–1296 (2014).
Ohman, E. M. et al. Clinically significant bleeding with low-dose rivaroxaban versus aspirin, in addition to P2Y12 inhibition, in acute coronary syndromes (GEMINI-ACS-1): a double-blind, multicentre, randomised trial. Lancet 389, 1799–1808 (2017).
Eikelboom, J. W. et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N. Engl. J. Med. 377, 1319–1330 (2017).
Vranckx, P. et al. Long-term ticagrelor monotherapy versus standard dual antiplatelet therapy followed by aspirin monotherapy in patients undergoing biolimus-eluting stent implantation: rationale and design of the GLOBAL LEADERS trial. EuroIntervention 12, 1239–1245 (2016).
Baber, U. et al. Ticagrelor with aspirin or alone in high-risk patients after coronary intervention: rationale and design of the TWILIGHT study. Am. Heart J. 182, 125–134 (2016).
Westein, E. et al. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc. Natl Acad. Sci. USA 110, 1357–1362 (2013).
Moake, J. L., Turner, N. A., Stathopoulos, N. A., Nolasco, L. H. & Hellums, J. D. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J. Clin. Invest. 78, 1456–1461 (1986).
Ruggeri, Z. M., Orje, J. N., Habermann, R., Federici, A. B. & Reininger, A. J. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 108, 1903–1910 (2006).
Konstantinides, S. et al. Distinct antithrombotic consequences of platelet glycoprotein Ibα and VI deficiency in a mouse model of arterial thrombosis. J. Thromb. Haemost. 4, 2014–2021 (2006).
Bergmeier, W. et al. The role of platelet adhesion receptor GPIbα far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc. Natl Acad. Sci. USA 103, 16900–16905 (2006).
Strassel, C. et al. Decreased thrombotic tendency in mouse models of the Bernard–Soulier syndrome. Arterioscler. Thromb. Vasc. Biol. 27, 241–247 (2007).
Wu, D., Meiring, M., Kotze, H. F., Deckmyn, H. & Cauwenberghs, N. Inhibition of platelet glycoprotein Ib, glycoprotein IIb/IIIa, or both by monoclonal antibodies prevents arterial thrombosis in baboons. Arterioscler. Thromb. Vasc. Biol. 22, 323–328 (2002).
Kageyama, S. et al. Anti-thrombotic effects and bleeding risk of AJvW-2, a monoclonal antibody against human von Willebrand factor. Br. J. Pharmacol. 122, 165–171 (1997).
Ulrichts, H. et al. Antithrombotic drug candidate ALX-0081 shows superior preclinical efficacy and safety compared with currently marketed antiplatelet drugs. Blood 118, 757–765 (2011).
Lei, X. et al. Anfibatide, a novel GPIb complex antagonist, inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis. Thromb. Haemost. 111, 279–289 (2014).
Diener, J. L. et al. Inhibition of von Willebrand factor-mediated platelet activation and thrombosis by the anti-von Willebrand factor A1-domain aptamer ARC1779. J. Thromb. Haemost. 7, 1155–1162 (2009).
Wadanoli, M. et al. The von Willebrand factor antagonist (GPG-290) prevents coronary thrombosis without prolongation of bleeding time. Thromb. Haemost. 98, 397–405 (2007).
Azzam, K., Garfinkel, L. I., Bal dit Sollier, C., Cisse Thiam, M. & Drouet, L. Antithrombotic effect of a recombinant von Willebrand factor, VCL, on nitrogen laser-induced thrombus formation in guinea pig mesenteric arteries. Thromb. Haemost. 73, 318–323 (1995).
Markus, H. S. et al. The von Willebrand inhibitor ARC1779 reduces cerebral embolization after carotid endarterectomy: a randomized trial. Stroke 42, 2149–2153 (2011).
Bartunek, J. et al. Novel antiplatelet agents: ALX-0081, a nanobody directed towards von Willebrand factor. J. Cardiovasc. Transl Res. 6, 355–363 (2013).
Gratacap, M. P. et al. Regulation and roles of PI3Kβ, a major actor in platelet signaling and functions. Adv. Enzyme Regul. 51, 106–116 (2011).
Martin, V. et al. Deletion of the p110β isoform of phosphoinositide 3-kinase in platelets reveals its central role in Akt activation and thrombus formation in vitro and in vivo. Blood 115, 2008–2013 (2010).
Nylander, S. et al. Human target validation of phosphoinositide 3-kinase (PI3K)β: effects on platelets and insulin sensitivity, using AZD6482 a novel PI3Kβ inhibitor. J. Thromb. Haemost. 10, 2127–2136 (2012).
Nylander, S., Wagberg, F., Andersson, M., Skarby, T. & Gustafsson, D. Exploration of efficacy and bleeding with combined phosphoinositide 3-kinase β inhibition and aspirin in man. J. Thromb. Haemost. 13, 1494–1502 (2015).
Laurent, P. A. et al. Platelet PI3Kβ and GSK3 regulate thrombus stability at a high shear rate. Blood 125, 881–888 (2015).
Giordanetto, F. et al. Discovery of 9-(1-phenoxyethyl)-2-morpholino-4-oxo-pyrido[1,2-a]pyrimidine-7- carboxamides as oral PI3Kβ inhibitors, useful as antiplatelet agents. Bioorg. Med. Chem. Lett. 24, 3936–3943 (2014).
Peter, K. et al. Induction of fibrinogen binding and platelet aggregation as a potential intrinsic property of various glycoprotein IIb/IIIa (αIIbβ3) inhibitors. Blood 92, 3240–3249 (1998).
Bassler, N. et al. A mechanistic model for paradoxical platelet activation by ligand-mimetic αIIbβ3 (GPIIb/IIIa) antagonists. Arterioscler. Thromb. Vasc. Biol. 27, e9–e15 (2007).
Peter, K. et al. Platelet activation as a potential mechanism of GP IIb/IIIa inhibitor-induced thrombocytopenia. Am. J. Cardiol. 84, 519–524 (1999).
Armstrong, P. C. & Peter, K. GPIIb/IIIa inhibitors: from bench to bedside and back to bench again. Thromb. Haemost. 107, 808–814 (2012).
Li, J. et al. RUC-4: a novel αIIbβ3 antagonist for prehospital therapy of myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 34, 2321–2329 (2014).
Schwarz, M. et al. Conformation-specific blockade of the integrin GPIIb/IIIa: a novel antiplatelet strategy that selectively targets activated platelets. Circ. Res. 99, 25–33 (2006).
Hohmann, J. D. et al. Delayed targeting of CD39 to activated platelet GPIIb/IIIa via a single-chain antibody: breaking the link between antithrombotic potency and bleeding? Blood 121, 3067–3075 (2013).
Stoll, P. et al. Targeting ligand-induced binding sites on GPIIb/IIIa via single-chain antibody allows effective anticoagulation without bleeding time prolongation. Arterioscler. Thromb. Vasc. Biol. 27, 1206–1212 (2007).
Wang, X. et al. Towards effective and safe thrombolysis and thromboprophylaxis: preclinical testing of a novel antibody-targeted recombinant plasminogen activator directed against activated platelets. Circ. Res. 114, 1083–1093 (2014).
Estevez, B., Shen, B. & Du, X. Targeting integrin and integrin signaling in treating thrombosis. Arterioscler. Thromb. Vasc. Biol. 35, 24–29 (2015).
Flaumenhaft, R. & De Ceunynck, K. Targeting PAR1: now what? Trends Pharmacol. Sci. 38, 701–716 (2017).
Aisiku, O. et al. Parmodulins inhibit thrombus formation without inducing endothelial injury caused by vorapaxar. Blood 125, 1976–1985 (2015).
Wong, P. C. et al. Blockade of protease-activated receptor-4 (PAR4) provides robust antithrombotic activity with low bleeding. Sci. Transl. Med. 9, eaaf5294 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02208882 (2015).
Flaumenhaft, R., Furie, B. & Zwicker, J. I. Therapeutic implications of protein disulfide isomerase inhibition in thrombotic disease. Arterioscler. Thromb. Vasc. Biol. 35, 16–23 (2015).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02195232 (2017).
Zahid, M. et al. The future of glycoprotein VI as an antithrombotic target. J. Thromb. Haemost. 10, 2418–2427 (2012).
Mangin, P. et al. Thrombin overcomes the thrombosis defect associated with platelet GPVI/FcRγ deficiency. Blood 107, 4346–4353 (2006).
Bender, M., Hagedorn, I. & Nieswandt, B. Genetic and antibody-induced glycoprotein VI deficiency equally protects mice from mechanically and FeCl3-induced thrombosis. J. Thromb. Haemost. 9, 1423–1426 (2011).
Ohlmann, P. et al. Ex vivo inhibition of thrombus formation by an anti-glycoprotein VI Fab fragment in non-human primates without modification of glycoprotein VI expression. J. Thromb. Haemost. 6, 1003–1011 (2008).
Muzard, J. et al. Design and humanization of a murine scFv that blocks human platelet glycoprotein VI in vitro. FEBS J. 276, 4207–4222 (2009).
Ungerer, M. et al. Novel antiplatelet drug revacept (Dimeric Glycoprotein VI-Fc) specifically and efficiently inhibited collagen-induced platelet aggregation without affecting general hemostasis in humans. Circulation 123, 1891–1899 (2011).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01645306 (2017).
Jamasbi, J. et al. Differential inhibition of human atherosclerotic plaque-induced platelet activation by dimeric GPVI-Fc and anti-GPVI antibodies: functional and imaging studies. J. Am. Coll. Cardiol. 65, 2404–2415 (2015).
Greene, T. K., Schiviz, A., Hoellriegl, W., Poncz, M. & Muchitsch, E. M. Towards a standardization of the murine tail bleeding model. J. Thromb. Haemost. 8, 2820–2822 (2010).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT00853450 (2009).
Acknowledgements
J.D.M. is supported by a Haematology Society of Australia and New Zealand New Investigator Scholarship. M.S. is supported by a fellowship from the French Foundation for Medical Research. K.P. is supported by a principal research fellowship from the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Contributions
All the authors researched data for the article, discussed its content, wrote the manuscript, and reviewed and edited it before submission.
Corresponding author
Ethics declarations
Competing interests
K.P. is an inventor on patents describing antiplatelet antibody compounds. The other authors declare no competing interests.
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
McFadyen, J., Schaff, M. & Peter, K. Current and future antiplatelet therapies: emphasis on preserving haemostasis. Nat Rev Cardiol 15, 181–191 (2018). https://doi.org/10.1038/nrcardio.2017.206
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2017.206
This article is cited by
-
Insights into platelet pharmacology from a cryo-EM structure of the ABCC4 transporter
Nature Cardiovascular Research (2023)
-
Cordycepin Inhibits Pathological High Shear-Induced Platelet Aggregation, Activation, and Phosphatidylserine Exposure by Regulating the Phosphorylation of Akt Protein
Revista Brasileira de Farmacognosia (2023)
-
Early Guideline-Directed Medical Therapy and in-Hospital Major Bleeding Risk in ST-Elevation Myocardial Infarction Patients Treated with Percutaneous Coronary Intervention: Findings from the CCC-ACS Project
Cardiovascular Drugs and Therapy (2023)
-
Three finger toxins of elapids: structure, function, clinical applications and its inhibitors
Molecular Diversity (2023)
-
An Updated Review on Glycoprotein IIb/IIIa Inhibitors as Antiplatelet Agents: Basic and Clinical Perspectives
High Blood Pressure & Cardiovascular Prevention (2023)