Key Points
-
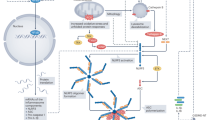
The inflammasome is a macromolecular structure in the cell responsible for sensing danger and triggering a local or systemic inflammatory response
-
Upon activation, the inflammasome produces large amounts of active cytokines (primarily IL-1β) for extracellular secretion, and those cytokines mediate the acute phase of an inflammatory response, such as fever
-
The most widely characterized inflammasome sensor in the heart is NACHT, LRR, and PYD domains-containing protein 3 (NLRP3), which is activated in response to noninfectious stimuli such as cell debris during acute myocardial infarction
-
Activation of the NLRP3 inflammasome triggers further myocardial damage indirectly through the release of IL-1β and directly through promotion of inflammatory cell death via pyroptosis
-
Experimental studies have shown that strategies inhibiting the activation of the NLRP3 inflammasome in the early reperfusion period after acute myocardial infarction reduce the overall size of the infarct and preserve normal cardiac function
-
IL-1 blockade can prevent the recurrence of acute myocardial infarction in patients who have experienced a previous event and might improve exercise capacity and cardiac function in patients with heart failure
Abstract
The heart is extremely sensitive to ischaemic injury. During an acute myocardial infarction (AMI) event, the injury is initially caused by reduced blood supply to the tissues, which is then further exacerbated by an intense and highly specific inflammatory response that occurs during reperfusion. Numerous studies have highlighted the central role of the NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome in this process. The inflammasome, an integral part of the innate immune system, is a macromolecular protein complex that finely regulates the activation of caspase 1 and the production and secretion of powerful pro-inflammatory cytokines such as IL-1β and IL-18. In this Review, we summarize evidence supporting the therapeutic value of NLRP3 inflammasome-targeted strategies in experimental models, and the data supporting the role of the NLRP3 inflammasome in AMI and its consequences on adverse cardiac remodelling, cytokine-mediated systolic dysfunction, and heart failure.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Anderson, J. L. & Morrow, D. A. Acute myocardial infarction. N. Engl. J. Med. 376, 2053–2064 (2017).
Eapen, Z. J. et al. Defining heart failure end points in ST-segment elevation myocardial infarction trials: integrating past experiences to chart a path forward. Circ. Cardiovasc. Qual. Outcomes 5, 594–600 (2012).
Seropian, I. M., Toldo, S., van Tassell, B. W. & Abbate, A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J. Am. Coll. Cardiol. 63, 1593–1603 (2014).
Westman, P. C. et al. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J. Am. Coll. Cardiol. 67, 2050–2060 (2016).
Gao, X. M., White, D. A., Dart, A. M. & Du, X. J. Post-infarct cardiac rupture: recent insights on pathogenesis and therapeutic interventions. Pharmacol. Ther. 134, 156–179 (2012).
Abbate, A. et al. Alterations in the interleukin-1/interleukin-1 receptor antagonist balance modulate cardiac remodeling following myocardial infarction in the mouse. PLoS ONE 6, e27923 (2011).
Savvatis, K. et al. Interleukin-23 deficiency leads to impaired wound healing and adverse prognosis after myocardial infarction. Circ. Heart Fail. 7, 161–171 (2014).
Toldo, S. et al. The inflammasome in myocardial injury and cardiac remodeling. Antioxid. Redox Signal. 22, 1146–1161 (2015).
Sutterwala, F. S., Haasken, S. & Cassel, S. L. Mechanism of NLRP3 inflammasome activation. Ann. NY Acad. Sci. 1319, 82–95 (2014).
Mezzaroma, E. et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl Acad. Sci. USA 108, 19725–19730 (2011).
Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206 (2014).
Dinarello, C. A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732 (2011).
Westermann, D. et al. Cardioprotective and anti-inflammatory effects of interleukin converting enzyme inhibition in experimental diabetic cardiomyopathy. Diabetes 56, 1834–1841 (2007).
Rider, P., Carmi, Y., Voronov, E. & Apte, R. N. Interleukin-1α. Semin. Immunol. 25, 430–438 (2013).
Gross, O. et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36, 388–400 (2012).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Shao, W., Yeretssian, G., Doiron, K., Hussain, S. N. & Saleh, M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J. Biol. Chem. 282, 36321–36329 (2007).
Toldo, S. et al. Independent roles of the priming and the triggering of the NLRP3 inflammasome in the heart. Cardiovasc. Res. 105, 203–212 (2015).
Kawaguchi, M. et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123, 594–604 (2011).
Liu, A., Gao, X., Zhang, Q. & Cui, L. Cathepsin B inhibition attenuates cardiac dysfunction and remodeling following myocardial infarction by inhibiting the NLRP3 pathway. Mol. Med. Rep. 8, 361–366 (2013).
He, Y., Zeng, M. Y., Yang, D., Motro, B. & Núñez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016).
Liu, Y. et al. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res. Cardiol. 109, 415 (2014).
Ito, M. et al. Bruton's tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 6, 7360 (2015).
Starkov, A. A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. NY Acad. Sci. 1147, 37–52 (2008).
Kim, J. S., He, L., Qian, T. & Lemasters, J. J. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr. Mol. Med. 3, 527–535 (2003).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Sadatomi, D. et al. Mitochondrial function is required for extracellular ATP-induced NLRP3 inflammasome activation. J. Biochem. 161, 503–512 (2017).
Sun, Q., Fan, J., Billiar, T. R. & Scott, M. J. Inflammasome and autophagy regulation — a two-way street. Mol. Med. http://dx.doi.org/10.2119/molmed.2017.00077 (2017).
Park, S. et al. Defective mitochondrial fission augments NLRP3 inflammasome activation. Sci. Rep. 5, 15489 (2015).
Mills, E. L., Kelly, B., O'Neill, L. A. J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 18, 488–498 (2017).
Iyer, S. S. et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323 (2013).
Shi, C. S. et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 13, 255–263 (2012).
Wu, X. et al. Impaired autophagy contributes to adverse cardiac remodeling in acute myocardial infarction. PLoS ONE 9, e112891 (2014).
Netea, M. G. et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood 113, 2324–2335 (2009).
Mezzaroma, E., Toldo, S. & Abbate, A. Role of NLRP3 (cryopyrin) in acute myocardial infarction. Cardiovasc. Res. 99, 225–226 (2013).
Wang, L., Qu, P., Zhao, J. & Chang, Y. NLRP3 and downstream cytokine expression elevated in the monocytes of patients with coronary artery disease. Arch. Med. Sci. 10, 791–800 (2014).
Toldo, S. et al. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int. J. Cardiol. 209, 215–220 (2016).
Sandanger, Ø. et al. NLRP3 inflammasome activation during myocardial ischemia reperfusion is cardioprotective. Biochem. Biophys. Res. Commun. 469, 1012–1020 (2016).
Marchetti, C. et al. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J. Cardiovasc. Pharmacol. 63, 316–322 (2014).
Mastrocola, R. et al. Pharmacological inhibition of NLRP3 inflammasome attenuates myocardial ischemia/reperfusion injury by activation of RISK and mitochondrial pathways. Oxid. Med. Cell. Longev. 2016, 5271251 (2016).
Takahashi, M. NLRP3 inflammasome as a novel player in myocardial infarction. Int. Heart J. 55, 101–105 (2014).
Mezzaroma, E., Marchetti, C. & Toldo, S. Letter by Mezzaroma, et al regarding article, “NLRP3 inflammasome as a therapeutic target in myocardial infarction”. Int. Heart J. 55, 379 (2014).
Li, X. et al. NOD2 deficiency protects against cardiac remodeling after myocardial infarction in mice. Cell. Physiol. Biochem. 32, 1857–1866 (2013).
Sandanger, Ø. et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 99, 164–174 (2013).
Jong, W. M. et al. Nlrp3 plays no role in acute cardiac infarction due to low cardiac expression. Int. J. Cardiol. 177, 41–43 (2014).
Lamkanfi, M. et al. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J. Cell Biol. 187, 61–70 (2009).
Marchetti, C. et al. Pharmacologic inhibition of the NLRP3 inflammasome preserves cardiac function after ischemic and nonischemic injury in the mouse. J. Cardiovasc. Pharmacol. 66, 1–8 (2015).
Marchetti, C., Swartzwelter, B., Koenders, M., Dinarello, C. A. & Joosten, L. A. OP0090 The human safe NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of experimental arthritis. Ann. Rheum. Dis. 76, 89 (2017).
OLATEC Lead compound. OLATEC http://www.olatec.com/lead-compound.html (2017).
Juliana, C. et al. Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 285, 9792–9802 (2010).
Kim, Y. S. et al. BAY 11–7082, a nuclear factor-κB inhibitor, reduces inflammation and apoptosis in a rat cardiac ischemia-reperfusion injury model. Int. Heart J. 51, 348–353 (2010).
Cocco, M. et al. Design, synthesis, and evaluation of acrylamide derivatives as direct NLRP3 inflammasome inhibitors. ChemMedChem. 11, 1790–1803 (2016).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
van Hout, G. P. et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur. Heart J. 38, 828–836 (2017).
Mauro, A. G., Thurber, C. & Abbate, A. Colchicine in acute myocardial infarction: “teaching new tricks to an old dog”. Transl Med. 5, e133 (2015).
Leung, Y. Y., Yao Hui, L. L. & Kraus, V. B. Colchicine — update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 45, 341–350 (2015).
Misawa, T. et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 14, 454–460 (2013).
Fujisue, K. et al. Colchicine improves survival, left ventricular remodeling, and chronic cardiac function after acute myocardial infarction. Circ. J. 81, 1174–1182 (2017).
Toldo, S., Marchetti, C. & Abbate, A. Re. “NLRP3 inflammasome activation during myocardial ischemia reperfusion is cardioprotective”. Biochem. Biophys. Res. Commun. 470, 811–812 (2016).
Sager, H. B. et al. Targeting interleukin-1β reduces leukocyte production after acute myocardial infarction. Circulation 132, 1880–1890 (2015).
van Tassell, B. W., Toldo, S., Mezzaroma, E. & Abbate, A. Targeting interleukin-1 in heart disease. Circulation 128, 1910–1923 (2013).
Abbate, A. et al. Anakinra a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation 117, 2670–2683 (2008).
Toldo, S. et al. Interleukin-1β blockade improves cardiac remodelling after myocardial infarction without interrupting the inflammasome in the mouse. Exp. Physiol. 98, 734–745 (2013).
Toldo, S. et al. Interleukin-1β blockade improves left ventricular systolic/diastolic function and restores contractility reserve in severe ischemic cardiomyopathy in the mouse. J. Cardiovasc. Pharmacol. 64, 1–6 (2014).
Lugrin, J. et al. Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. J. Immunol. 194, 499–503 (2015).
Mauro, A. G. et al. Reduction of myocardial ischemia-reperfusion injury by inhibiting interleukin-1 alpha. J. Cardiovasc. Pharmacol. 69, 156–160 (2017).
O'Brien, L. C. et al. Interleukin-18 as a therapeutic target in acute myocardial infarction and heart failure. Mol. Med. 20, 221–229 (2014).
Pomerantz, B. J., Reznikov, L. L., Harken, A. H. & Dinarello, C. A. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1β. Proc. Natl Acad. Sci. USA 98, 2871–2876 (2001).
Venkatachalam, K. et al. Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J. Biol. Chem. 284, 7853–7865 (2009).
Gu, H. et al. The protective role of interleukin-18 binding protein in a murine model of cardiac ischemia/reperfusion injury. Transpl. Int. 28, 1436–1444 (2015).
Pörksen, G. et al. Periodic fever, mild arthralgias, and reversible moderate and severe organ inflammation associated with the V198M mutation in the CIAS1 gene in three German patients — expanding phenotype of CIAS1 related autoinflammatory syndrome. Eur. J. Haematol. 73, 123–127 (2004).
Kumar, A. et al. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J. Exp. Med. 183, 949–958 (1996).
Van Tassell, B. W. et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS ONE 7, e33438 (2012).
Van Tassell, B. W., Seropian, I. M., Toldo, S., Mezzaroma, E. & Abbate, A. Interleukin-1β induces a reversible cardiomyopathy in the mouse. Inflamm Res. 62, 637–640 (2013).
Zhang, W. et al. Cardiac fibroblasts contribute to myocardial dysfunction in mice with sepsis: the role of NLRP3 inflammasome activation. PLoS ONE 9, e107639 (2014).
Bracey, N. A. et al. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1β. Exp. Physiol. 98, 462–472 (2013).
Abbate, A. The heart on fire: inflammasome and cardiomyopathy. Exp. Physiol. 98, 385 (2013).
Wang, Y., Gao, B. & Xiong, S. Involvement of NLRP3 inflammasome in CVB3-induced viral myocarditis. Am. J. Physiol. Heart Circ. Physiol. 307, H1438–H1447 (2014).
Li, R. et al. Triptolide attenuates pressure overload-induced myocardial remodeling in mice via the inhibition of NLRP3 inflammasome expression. Biochem. Biophys. Res. Commun. 485, 69–75 (2017).
Seropian, I. M., Sonnino, C., Van Tassell, B. W., Biasucci, L. M. & Abbate, A. Inflammatory markers in ST-elevation acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 5, 382–395 (2016).
Deftereos, S. et al. Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation 132, 1395–1403 (2015).
Nidorf, S. M., Eikelboom, J. W., Budgeon, C. A. & Thompson, P. L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 61, 404–410 (2013).
Abbate, A. et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am. J. Cardiol. 105, 1371–1377 (2010).
Abbate, A. et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am. J. Cardiol. 111, 1394–1400 (2013).
Abbate, A. et al. Comparative safety of Interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am. J. Cardiol. 115, 288–292 (2015).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01950299 (2017).
Morton, A. C. et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur. Heart J. 36, 377–384 (2015).
Abbate, A. & Dinarello, C. A. Anti-inflammatory therapies in acute coronary syndromes: is IL-1 blockade a solution? Eur. Heart J. 36, 337–339 (2015).
Van Tassell, B. W. et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 113, 321–327 (2014).
Van Tassell, B. W. et al. Interleukin-1 blockade in acute decompensated heart failure: a randomized, double-blinded, placebo-controlled pilot study. J. Cardiovasc. Pharmacol. 67, 544–551 (2016).
Van Tassell, B. et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from the REcently Decompensated Heart failure Anakinra Response trial (REDHART). Circ. Heart Fail. (in press).
Van Tassell, B. et al. Interleukin-1 blockade in heart failure with preserved ejection fraction: rationale and design of the Diastolic Heart failure Anakinra Response Trial 2 (DHART2). Clin. Cardiol. 40, 626–632 (2017).
ILARIS. Learn how prescription ILARIS may help. ILARIS http://www.ilaris.com/index.jsp (2017).
Ridker, P. M., Thuren, T., Zalewski, A. & Libby, P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 162, 597–605 (2011).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Acknowledgements
The authors are grateful to Salvatore Carbone (Virginia Commonwealth University, Richmond, USA) for critically reviewing the manuscript and to Charles Dinarello (University of Colorado Denver, USA) for mentorship and guidance in the field of IL-1 over the past 10 years. S.T. is supported by a grant from the Virginia Commonwealth University Center for Clinical & Translational Research, a VCU Commercialization Fund Award, and a Department of Internal Medicine Pilot Study Award. A.A. is supported by grants (HL121402 and HL136816) from the National Heart, Lung, and Blood Institute, Bethesda, Maryland, USA.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, discussed the content, wrote the manuscript, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.T. has received research grants from Olatec. A.A. has received research grants from Novartis and Swedish Orphan Biovitrum and has served as a scientific adviser to Olatec.
Rights and permissions
About this article
Cite this article
Toldo, S., Abbate, A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 15, 203–214 (2018). https://doi.org/10.1038/nrcardio.2017.161
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2017.161
This article is cited by
-
Coenzyme Q10 mitigates macrophage mediated inflammation in heart following myocardial infarction via the NLRP3/IL1β pathway
BMC Cardiovascular Disorders (2024)
-
The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases
Nature Reviews Cardiology (2024)
-
Cardiac cell senescence: molecular mechanisms, key proteins and therapeutic targets
Cell Death Discovery (2024)
-
Immunomodulation and immunopharmacology in heart failure
Nature Reviews Cardiology (2024)
-
The E3 ubiquitin ligase MARCH2 protects against myocardial ischemia-reperfusion injury through inhibiting pyroptosis via negative regulation of PGAM5/MAVS/NLRP3 axis
Cell Discovery (2024)