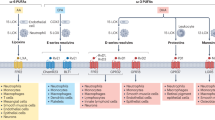
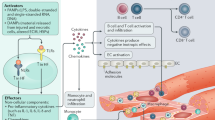
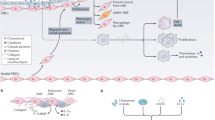
Key Points
-
Inflammation and its failure to resolve are firmly established as central to the development and complications of several cardiovascular diseases
-
Targeting of inflammatory processes in experimental models has been demonstrated to be beneficial in attenuating myocardial and arterial injury, reducing disease progression, and promoting healing, but clinical translation has been disappointing
-
Current tools to measure 'inflammation' are nonspecific and represent downstream sequelae of biological processes, but provide little insight into disease state, site, or activation pathways
-
Contemporary molecular techniques (such as proteomics and gene-expression analysis) improve our ability to characterize underlying biological processes, and identify activation pathways as biomarkers and as a basis to develop new therapeutics
-
Noninvasive imaging tools enable the identification of activation of specific pathways and their sites, and can be used to monitor response to therapy
Abstract
Inflammatory processes are firmly established as central to the development and complications of cardiovascular diseases. Elevated levels of inflammatory markers have been shown to be predictive of future cardiovascular events. The specific targeting of these processes in experimental models has been shown to attenuate myocardial and arterial injury, reduce disease progression, and promote healing. However, the translation of these observations and the demonstration of clear efficacy in clinical practice have been disappointing. A major limitation might be that tools currently used to measure 'inflammation' are insufficiently precise and do not provide information about disease site and activity, or discriminate between functionally important activation pathways. The challenge, therefore, is to make measures of inflammation that are more meaningful, and which can guide specific targeted therapies. In this Review, we consider the roles of inflammatory processes in the related pathologies of atherosclerosis and acute myocardial infarction, by providing an evaluation of the known and emerging inflammatory pathways. We highlight contemporary techniques to characterize and quantify inflammation, and consider how they might be used to guide specific treatments. Finally, we discuss emerging opportunities in the field, including their current limitations and challenges that are the focus of ongoing study.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
16 March 2017
In the version of this article initially published online and in print, there were some inaccuracies in Table 1. These errors have been corrected for the HTML and PDF versions of the article.
References
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 (2005).
Frangogiannis, N. G. Regulation of the inflammatory response in cardiac repair. Circ. Res. 110, 159–173 (2012).
Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 83, 456S–460S (2006).
Biasucci, L. M. et al. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation 99, 2079–2084 (1999).
Valgimigli, M. et al. Tumor necrosis factor-α receptor 1 is a major predictor of mortality and new-onset heart failure in patients with acute myocardial infarction: the Cytokine-Activation and Long-Term Prognosis in Myocardial Infarction (C-ALPHA) study. Circulation 111, 863–870 (2005).
Ridker, P. M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107, 363–369 (2003).
Nahrendorf, M., Pittet, M. J. & Swirski, F. K. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445 (2010).
Leuschner, F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 29, 1005–1010 (2011).
Panizzi, P. et al. Impaired infarct healing in atherosclerotic mice with Ly-6Chi monocytosis. J. Am. Coll. Cardiol. 55, 1629–1638 (2010).
Moore, K. J., Sheedy, F. J. & Fisher, E. A. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13, 709–721 (2013).
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866 (2014).
Jia, H. et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 62, 1748–1758 (2013).
Prondzinsky, R. et al. Interleukin-6, -7, -8 and -10 predict outcome in acute myocardial infarction complicated by cardiogenic shock. Clin. Res. Cardiol. 101, 375–384 (2012).
Dimitrijevic, O., Stojcevski, B. D., Ignjatovic, S. & Singh, N. M. Serial measurements of C-reactive protein after acute myocardial infarction in predicting one-year outcome. Int. Heart J. 47, 833–842 (2006).
von Hundelshausen, P. & Weber, C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ. Res. 100, 27–40 (2007).
Vinten-Johansen, J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 61, 481–497 (2004).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Nourshargh, S., Renshaw, S. A. & Imhof, B. A. Reverse migration of neutrophils: where, when, how, and why? Trends Immunol. 37, 273–286 (2016).
Yan, X. et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 62, 24–35 (2013).
Martin, J. L., Mestril, R., Hilal-Dandan, R., Brunton, L. L. & Dillmann, W. H. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation 96, 4343–4348 (1997).
Andrassy, M. et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117, 3216–3226 (2008).
Jiang, D. et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 11, 1173–1179 (2005).
Schoneveld, A. H. et al. Atherosclerotic lesion development and Toll like receptor 2 and 4 responsiveness. Atherosclerosis 197, 95–104 (2008).
Beg, A. A. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 23, 509–512 (2002).
Frantz, S. et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J. Clin. Invest. 104, 271–280 (1999).
Oyama, J. et al. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109, 784–789 (2004).
Riad, A. et al. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J. Immunol. 180, 6954–6961 (2008).
Satoh, M. et al. Activated toll-like receptor 4 in monocytes is associated with heart failure after acute myocardial infarction. Int. J. Cardiol. 109, 226–234 (2006).
Shishido, T. et al. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation 108, 2905–2910 (2003).
Ruparelia, N. et al. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur. Heart J. 36, 1923–1934 (2015).
Takahashi, M. NLRP3 inflammasome as a novel player in myocardial infarction. Int. Heart J. 55, 101–105 (2014).
Mezzaroma, E. et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl Acad. Sci. USA 108, 19725–19730 (2011).
Dreyer, W. J. et al. Kinetics of C5a release in cardiac lymph of dogs experiencing coronary artery ischemia-reperfusion injury. Circ. Res. 71, 1518–1524 (1992).
Birdsall, H. H. et al. Complement C5a, TGF-β1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation 95, 684–692 (1997).
Distelmaier, K. et al. Local complement activation triggers neutrophil recruitment to the site of thrombus formation in acute myocardial infarction. Thromb. Haemost. 102, 564–572 (2009).
Torzewski, J. et al. Complement-induced release of monocyte chemotactic protein-1 from human smooth muscle cells: a possible initiating event in atherosclerotic lesion formation. Arterioscler. Thromb. Vasc. Biol. 16, 673–677 (1996).
Lakshminarayanan, V. et al. Reactive oxygen intermediates induce monocyte chemotactic protein-1 in vascular endothelium after brief ischemia. Am. J. Pathol. 159, 1301–1311 (2001).
Sellak, H., Franzini, E., Hakim, J. & Pasquier, C. Reactive oxygen species rapidly increase endothelial ICAM-1 ability to bind neutrophils without detectable upregulation. Blood 83, 2669–2677 (1994).
Shingu, M. et al. Activation of complement in normal serum by hydrogen peroxide and hydrogen peroxide-related oxygen radicals produced by activated neutrophils. Clin. Exp. Immunol. 90, 72–78 (1992).
Maekawa, N. et al. Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-α. J. Am. Coll. Cardiol. 39, 1229–1235 (2002).
Kurrelmeyer, K. M. et al. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc. Natl Acad. Sci. USA 97, 5456–5461 (2000).
Saxena, A. et al. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J. Immunol. 191, 4838–4848 (2013).
Miyao, Y. et al. Elevated plasma interleukin-6 levels in patients with acute myocardial infarction. Am. Heart J. 126, 1299–1304 (1993).
Dawn, B. et al. IL-6 plays an obligatory role in late preconditioning via JAK–STAT signaling and upregulation of iNOS and COX-2. Cardiovasc. Res. 64, 61–71 (2004).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Kim, E. J., Kim, S., Kang, D. O. & Seo, H. S. Metabolic activity of the spleen and bone marrow in patients with acute myocardial infarction evaluated by 18f-fluorodeoxyglucose positron emission tomograpic imaging. Circ. Cardiovasc. Imaging 7, 454–460 (2014).
Dutta, P. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012).
Weinberger, T. & Schulz, C. Myocardial infarction: a critical role of macrophages in cardiac remodeling. Front. Physiol. 6, 107 (2015).
Tsujioka, H. et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J. Am. Coll. Cardiol. 54, 130–138 (2009).
Horckmans, M. et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J. http://dx.doi.org/10.1093/eurheartj/ehw002 (2016).
Levick, S. P. et al. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc. Res. 89, 12–19 (2011).
Hofmann, U. et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125, 1652–1663 (2012).
Liuzzo, G. et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 101, 2883–2888 (2000).
Ammirati, E. et al. Expansion of T-cell receptor ζdim effector T cells in acute coronary syndromes. Arterioscler. Thromb. Vasc. Biol. 28, 2305–2311 (2008).
Zal, B. et al. Heat-shock protein 60-reactive CD4+CD28null T cells in patients with acute coronary syndromes. Circulation 109, 1230–1235 (2004).
Liuzzo, G. et al. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J. Am. Coll. Cardiol. 50, 1450–1458 (2007).
Mor, A., Luboshits, G., Planer, D., Keren, G. & George, J. Altered status of CD4+CD25+ regulatory T cells in patients with acute coronary syndromes. Eur. Heart J. 27, 2530–2537 (2006).
Blancke, F. et al. Systemic inflammation and reperfusion injury in patients with acute myocardial infarction. Mediators Inflamm. 2005, 385–389 (2005).
Kohsaka, S. et al. Systemic inflammatory response syndrome after acute myocardial infarction complicated by cardiogenic shock. Arch. Intern. Med. 165, 1643–1650 (2005).
Debrunner, M. et al. Proinflammatory cytokines in acute myocardial infarction with and without cardiogenic shock. Clin. Res. Cardiol. 97, 298–305 (2008).
Leuschner, F. et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ. Res. 107, 1364–1373 (2010).
Majmudar, M. D. et al. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ. Res. 112, 755–761 (2013).
Pearson, T. A. et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107, 499–511 (2003).
Johnson, B. D. et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation 109, 726–732 (2004).
Danesh, J. et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 350, 1387–1397 (2004).
Haim, M., Boyko, V., Goldbourt, U., Battler, A. & Behar, S. Predictive value of elevated white blood cell count in patients with preexisting coronary heart disease: the Bezafibrate Infarction Prevention Study. Arch. Intern. Med. 164, 433–439 (2004).
Lee, C. D. et al. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am. J. Epidemiol. 154, 758–764 (2001).
Berk, B. C., Weintraub, W. S. & Alexander, R. W. Elevation of C-reactive protein in “active” coronary artery disease. Am. J. Cardiol. 65, 168–172 (1990).
Ridker, P. M., Cushman, M., Stampfer, M. J., Tracy, R. P. & Hennekens, C. H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 336, 973–979 (1997).
Ridker, P. M., Cushman, M., Stampfer, M. J., Tracy, R. P. & Hennekens, C. H. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation 97, 425–428 (1998).
Zacho, J. et al. Genetically elevated C-reactive protein and ischemic vascular disease. N. Engl. J. Med. 359, 1897–1908 (2008).
Hirschfield, G. M. et al. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc. Natl Acad. Sci. USA 102, 8309–8314 (2005).
Passlick, B., Flieger, D. & Ziegler-Heitbrock, H. W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74, 2527–2534 (1989).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Deng, M. C. et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am. J. Transplant. 6, 150–160 (2006).
Golub, T. R. et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 286, 531–537 (1999).
van't Veer, L. J. et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530–536 (2002).
Bendall, S. C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Haas, B. et al. Proteomic analysis of plasma samples from patients with acute myocardial infarction identifies haptoglobin as a potential prognostic biomarker. J. Proteomics 75, 229–236 (2011).
Shah, S. H. & Newgard, C. B. Integrated metabolomics and genomics: systems approaches to biomarkers and mechanisms of cardiovascular disease. Circ. Cardiovasc. Genet. 8, 410–419 (2015).
Stegemann, C. et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 129, 1821–1831 (2014).
Malik, Z. A. et al. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am. J. Physiol. Heart Circ. Physiol. 304, H954–H965 (2013).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Muralidharan-Chari, V., Clancy, J. W., Sedgwick, A. & D'Souza-Schorey, C. Microvesicles: mediators of extracellular communication during cancer progression. J. Cell Sci. 123, 1603–1611 (2010).
Wickman, G. R. et al. Blebs produced by actin–myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ. 20, 1293–1305 (2013).
Wickman, G., Julian, L. & Olson, M. F. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 19, 735–742 (2012).
Tans, G. et al. Comparison of anticoagulant and procoagulant activities of stimulated platelets and platelet-derived microparticles. Blood 77, 2641–2648 (1991).
Taraboletti, G. et al. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 160, 673–680 (2002).
Boulanger, C. M. et al. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 104, 2649–2652 (2001).
Deng, Z. B. et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 58, 2498–2505 (2009).
Mesri, M. & Altieri, D. C. Endothelial cell activation by leukocyte microparticles. J. Immunol. 161, 4382–4387 (1998).
Martin, S. et al. Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation 109, 1653–1659 (2004).
Quiat, D. & Olson, E. N. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J. Clin. Invest. 123, 11–18 (2013).
Zhang, Y. et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell 39, 133–144 (2010).
Bye, A. et al. Circulating microRNAs predict future fatal myocardial infarction in healthy individuals — the HUNT study. J. Mol. Cell. Cardiol. 97, 162–168 (2016).
Yilmaz, A. et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur. Heart J. 34, 462–475 (2013).
Alam, S. R. et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circ. Cardiovasc. Imaging 5, 559–565 (2012).
Lee, W. W. et al. PET/MRI of inflammation in myocardial infarction. J. Am. Coll. Cardiol. 59, 153–163 (2012).
Jung, K. et al. Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ. Res. 112, 891–899 (2013).
Wollenweber, T. et al. Characterizing the inflammatory tissue response to acute myocardial infarction by clinical multimodality noninvasive imaging. Circ. Cardiovasc. Imaging 7, 811–818 (2014).
Rischpler, C. et al. Prospective evaluation of 18F-fluorodeoxyglucose uptake in postischemic myocardium by simultaneous positron emission tomography/magnetic resonance imaging as a prognostic marker of functional outcome. Circ. Cardiovasc. Imaging 9, e004316 (2016).
McAteer, M. A. et al. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat. Med. 13, 1253–1258 (2007).
McAteer, M. A. et al. A leukocyte-mimetic magnetic resonance imaging contrast agent homes rapidly to activated endothelium and tracks with atherosclerotic lesion macrophage content. Arterioscler. Thromb. Vasc. Biol. 32, 1427–1435 (2012).
Akhtar, A. M. et al. In vivo quantification of VCAM-1 expression in renal ischemia reperfusion injury using non-invasive magnetic resonance molecular imaging. PLoS ONE 5, e12800 (2010).
Sahul, Z. H. et al. Targeted imaging of the spatial and temporal variation of matrix metalloproteinase activity in a porcine model of postinfarct remodeling: relationship to myocardial dysfunction. Circ. Cardiovasc. Imaging 4, 381–391 (2011).
Ross, R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Choudhury, R. P., Lee, J. M. & Greaves, D. R. Mechanisms of disease: macrophage-derived foam cells emerging as therapeutic targets in atherosclerosis. Nat. Clin. Pract. Cardiovasc. Med. 2, 309–315 (2005).
Tabas, I., Williams, K. J. & Boren, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 116, 1832–1844 (2007).
van Furth, R. & Cohn, Z. A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 128, 415–435 (1968).
Cybulsky, M. I. & Gimbrone, M. A. Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251, 788–791 (1991).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Combadiere, C. et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117, 1649–1657 (2008).
Heidt, T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758 (2014).
Ley, K., Miller, Y. I. & Hedrick, C. C. Monocyte and macrophage dynamics during atherogenesis. Arterioscler. Thromb. Vasc. Biol. 31, 1506–1516 (2011).
Robbins, C. S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
Khallou-Laschet, J. et al. Macrophage plasticity in experimental atherosclerosis. PLoS ONE 5, e8852 (2010).
Schissel, S. L. et al. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J. Clin. Invest. 98, 1455–1464 (1996).
Barthwal, M. K. et al. Fluid-phase pinocytosis of native low density lipoprotein promotes murine M-CSF differentiated macrophage foam cell formation. PLoS ONE 8, e58054 (2013).
Shao, B. Z., Han, B. Z., Zeng, Y. X., Su, D. F. & Liu, C. The roles of macrophage autophagy in atherosclerosis. Acta Pharmacol. Sin. 37, 150–156 (2016).
Chan, K. F., Siegel, M. R. & Lenardo, J. M. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity 13, 419–422 (2000).
Ait-Oufella, H., Taleb, S., Mallat, Z. & Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31, 969–979 (2011).
Gharavi, N. M. et al. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. J. Biol. Chem. 282, 31460–31468 (2007).
Michel, J. B., Virmani, R., Arbustini, E. & Pasterkamp, G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur. Heart J. 32, 1977–1985 (2011).
Hellings, W. E. et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation 121, 1941–1950 (2010).
Vink, A. et al. HIF-1α expression is associated with an atheromatous inflammatory plaque phenotype and upregulated in activated macrophages. Atherosclerosis 195, e69–e75 (2007).
Sluimer, J. C. & Daemen, M. J. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J. Pathol. 218, 7–29 (2009).
Parathath, S. et al. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ. Res. 109, 1141–1152 (2011).
Ugocsai, P. et al. HIF-1beta determines ABCA1 expression under hypoxia in human macrophages. Int. J. Biochem. Cell Biol. 42, 241–252 (2010).
Folco, E. et al. Hypoxia but not inflammation augments glucose uptake in human macrophages J. Am. Coll. Cardiol. 58, 603–614 (2011).
Rong, J. X., Shapiro, M., Trogan, E. & Fisher, E. A. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc. Natl Acad. Sci. USA 100, 13531–13536 (2003).
Feil, S. et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ. Res. 115, 662–667 (2014).
Shankman, L. S. et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 21, 628–637 (2015).
Allahverdian, S., Chehroudi, A. C., McManus, B. M., Abraham, T. & Francis, G. A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 129, 1551–1559 (2014).
Vengrenyuk, Y. et al. Cholesterol loading reprograms the microRNA-143/145–myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler. Thromb. Vasc. Biol. 35, 535–546 (2015).
Williams, K. J., Feig, J. E. & Fisher, E. A. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat. Clin. Pract. Cardiovasc. Med. 5, 91–102 (2008).
Nissen, S. E. et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 352, 29–38 (2005).
Ridker, P. M. et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207 (2008).
Semaan, H. B. et al. Soluble VCAM-1 and E-selectin, but not ICAM-1 discriminate endothelial injury in patients with documented coronary artery disease. Cardiology 93, 7–10 (2000).
Hwang, S. J. et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation 96, 4219–4225 (1997).
Blann, A. D., Lip, G. Y., Beevers, D. G. & McCollum, C. N. Soluble P-selectin in atherosclerosis: a comparison with endothelial cell and platelet markers. Thromb. Haemost. 77, 1077–1080 (1997).
Swirski, F. K., Weissleder, R. & Pittet, M. J. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29, 1424–1432 (2009).
Schlitt, A. et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-α levels. Thromb. Haemost. 92, 419–424 (2004).
Poitou, C. et al. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31, 2322–2330 (2011).
Feig, J. E. et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 123, 989–998 (2011).
Lee, K. et al. Increased expression of fatty acid binding protein 4 and leptin in resident macrophages characterises atherosclerotic plaque rupture. Atherosclerosis 226, 74–81 (2013).
Puig, O. et al. A gene expression signature that classifies human atherosclerotic plaque by relative inflammation status. Circ. Cardiovasc. Genet. 4, 595–604 (2011).
Hulsmans, M. & Holvoet, P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc. Res. 100, 7–18 (2013).
Rautou, P. E. et al. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ. Res. 108, 335–343 (2011).
Skajaa, T. et al. High-density lipoprotein-based contrast agents for multimodal imaging of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30, 169–176 (2010).
Ruehm, S. G., Corot, C., Vogt, P., Cristina, H. & Debatin, J. F. Ultrasmall superparamagnetic iron oxide-enhanced MR imaging of atherosclerotic plaque in hyperlipidemic rabbits. Acad. Radiol. 9 (Suppl. 1), S143–S144 (2002).
Tarkin, J. M., Joshi, F. R. & Rudd, J. H. PET imaging of inflammation in atherosclerosis. Nat. Rev. Cardiol. 11, 443–457 (2014).
Moon, S. H. et al. Carotid FDG uptake improves prediction of future cardiovascular events in asymptomatic individuals. JACC Cardiovasc. Imaging 8, 949–956 (2015).
Joshi, N. V. et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 383, 705–713 (2014).
Nahrendorf, M. et al. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscler. Thromb. Vasc. Biol. 29, 1444–1451 (2009).
Vinegoni, C. et al. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci. Transl Med. 3, 84ra45 (2011).
Lee, S. et al. Fully integrated high-speed intravascular optical coherence tomography/near-infrared fluorescence structural/molecular imaging in vivo using a clinically available near-infrared fluorescence-emitting indocyanine green to detect inflamed lipid-rich atheromata in coronary-sized vessels. Circ. Cardiovasc. Interv. 7, 560–569 (2014).
Ughi, G. J. et al. Clinical characterization of coronary atherosclerosis with dual-modality OCT and near-infrared autofluorescence imaging. JACC Cardiovasc. Imaging http://dx.doi.org/10.1016/j.jcmg.2015.11.020 (2016).
Santos, R. et al. Dynamics of interferon-β modulated mRNA biomarkers in multiple sclerosis patients with anti-interferon-β neutralizing antibodies. J. Neuroimmunol. 176, 125–133 (2006).
Yang, Y., Blomme, E. A. & Waring, J. F. Toxicogenomics in drug discovery: from preclinical studies to clinical trials. Chem. Biol. Interact. 150, 71–85 (2004).
Cardilo-Reis, L. et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 4, 1072–1086 (2012).
Fredman, G. et al. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl Med. 7, 275ra20 (2015).
Barrett, P. M. & Topol, E. J. Pharmacogenetics: point-of-care genetic testing — a new frontier explored. Nat. Rev. Cardiol. 9, 315–316 (2012).
Kobara, M. et al. Antibody against interleukin-6 receptor attenuates left ventricular remodelling after myocardial infarction in mice. Cardiovasc. Res. 87, 424–430 (2010).
Kleveland, O. et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur. Heart J. 37, 2406–2413 (2016).
Toldo, S. et al. Interleukin-1β blockade improves cardiacremodelling after myocardial infarction without interrupting the inflammasome in the mouse. Exp. Physiol. 98, 734–745 (2013).
Hwang, M. W. et al. Neutralization of interleukin-1β in the acute phase of myocardial infarction promotes the progression of left ventricular remodeling. J. Am. Coll. Cardiol. 38, 1546–1553 (2001).
Abbate, A. et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation 117, 2670–2683 (2008).
Morton, A. C. et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur. Heart J. 36, 377–384 (2015).
Abbate, A. et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am. J. Cardiol. 115, 288–292 (2015).
Sugano, M., Tsuchida, K., Hata, T. & Makino, N. In vivo transfer of soluble TNF-alpha receptor 1 gene improves cardiac function and reduces infarct size after myocardial infarction in rats. FASEB J. 18, 911–913 (2004).
Padfield, G. J. et al. Cardiovascular effects of tumour necrosis factor α antagonism in patients with acute myocardial infarction: a first in human study. Heart 99, 1330–1335 (2013).
Matsumura, S. et al. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J. Clin. Invest. 115, 599–609 (2005).
Hudson, M. P. et al. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J. Am. Coll. Cardiol. 48, 15–20 (2006).
Ducharme, A. et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J. Clin. Invest. 106, 55–62 (2000).
Cerisano, G. et al. Early short-term doxycycline therapy in patients with acute myocardial infarction and left ventricular dysfunction to prevent the ominous progression to adverse remodelling: the TIPTOP trial. Eur. Heart J. 35, 184–191 (2014).
Slagman, A. C. et al. Specific removal of C-reactive protein by apheresis in a porcine cardiac infarction model. Blood Purif. 31, 9–17 (2011).
Wang, K. et al. Recombinant soluble P-selectin glycoprotein ligand-Ig (rPSGL-Ig) attenuates infarct size and myeloperoxidase activity in a canine model of ischemia-reperfusion. Thromb. Haemost. 88, 149–154 (2002).
Tardif, J. C. et al. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: results of the SELECT-ACS trial. J. Am. Coll. Cardiol. 61, 2048–2055 (2013).
Toldo, S. et al. Alpha-1 antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 51, 244–251 (2011).
Abbate, A. et al. Effects of Prolastin C (Plasma-Derived Alpha-1 Antitrypsin) on the acute inflammatory response in patients with ST-segment elevation myocardial infarction (from the VCU-alpha 1-RT pilot study). Am. J. Cardiol. 115, 8–12 (2015).
Weisman, H. F. et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 249, 146–151 (1990).
Investigators, A. A. et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA 297, 43–51 (2007).
Arai, M. et al. An anti-CD18 antibody limits infarct size and preserves left ventricular function in dogs with ischemia and 48-hour reperfusion. J. Am. Coll. Cardiol. 27, 1278–1285 (1996).
Aversano, T., Zhou, W., Nedelman, M., Nakada, M. & Weisman, H. A chimeric IgG4 monoclonal antibody directed against CD18 reduces infarct size in a primate model of myocardial ischemia and reperfusion. J. Am. Coll. Cardiol. 25, 781–788 (1995).
Faxon, D. P. et al. The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study. J. Am. Coll. Cardiol. 40, 1199–1204 (2002).
Baran, K. W. et al. Double-blind, randomized trial of an anti-CD18 antibody in conjunction with recombinant tissue plasminogen activator for acute myocardial infarction: limitation of myocardial infarction following thrombolysis in acute myocardial infarction (LIMIT AMI) study. Circulation 104, 2778–2783 (2001).
Gurantz, D. et al. Etanercept or intravenous immunoglobulin attenuates expression of genes involved in post-myocardial infarction remodeling. Cardiovasc. Res. 67, 106–115 (2005).
Gullestad, L. et al. Intravenous immunogloblin does not reduce left ventricular remodelling in patients with myocardial dysfunction during hospitalization after acute myocardial infarction. Int. J. Cardiol. 168, 212–218 (2013).
O'Donoghue, M. L. et al. Effect of losmapimod on cardiovascular outcomes in patients hospitalized with acute myocardial infarction: a randomized clinical trial. JAMA 315, 1591–1599 (2016).
Elhage, R. et al. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation 97, 242–244 (1998).
Ridker, P. M. et al. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinuman Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 162, 597–605 (2011).
Choudhury, R. P. et al. Arterial effects of canakinumab in patients with atherosclerosis and type 2 diabetes mellitus of glucose intolerance. J. Am. Coll. Cardiol. 68, 1769–1780 (2016).
Devlin, C. M., Kuriakose, G., Hirsch, E. & Tabas, I. Genetic alterations of IL-1 receptor antagonist in mice affect plasma cholesterol level and foam cell lesion size. Proc. Natl Acad. Sci. USA 99, 6280–6285 (2002).
Branen, L. et al. Inhibition of tumor necrosis factor-α reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 24, 2137–2142 (2004).
Maki-Petaja, K. M. et al. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 126, 2473–2480 (2012).
Davenport, P. & Tipping, P. G. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 163, 1117–1125 (2003).
Papp, K. A. et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br. J. Dermatol. 168, 844–854 (2013).
Erbel, C. et al. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J. Immunol. 183, 8167–8175 (2009).
van de Kerkhof, P. C. et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J. Am. Acad. Dermatol. 75, 83–98.e4 (2016).
Jagavelu, K. et al. Systemic deficiency of the MAP kinase-activated protein kinase 2 reduces atherosclerosis in hypercholesterolemic mice. Circ. Res. 101, 1104–1112 (2007).
Elkhawad, M. et al. Effects of p38 mitogen-activated protein kinase inhibition on vascular and systemic inflammation in patients with atherosclerosis. JACC Cardiovasc. Imaging 5, 911–922 (2012).
Bursill, C. A., Choudhury, R. P., Ali, Z., Greaves, D. R. & Channon, K. M. Broad-spectrum CC-chemokine blockade by gene transfer inhibits macrophage recruitment and atherosclerotic plaque formation in apolipoprotein E-knockout mice. Circulation 110, 2460–2466 (2004).
Dong, Z. M. et al. The combined role of P- and E-selectins in atherosclerosis. J. Clin. Invest. 102, 145–152 (1998).
Stahli, B. E. et al. Effects of P-selectin antagonist inclacumab in patients undergoing coronary artery bypass graft surgery: SELECT-CABG Trial. J. Am. Coll. Cardiol. 67, 344–346 (2016).
Park, J. G. et al. Evaluation of VCAM-1 antibodies as therapeutic agent for atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 226, 356–363 (2013).
Fraser, H. et al. Varespladib (A-002), a secretory phospholipase A2 inhibitor, reduces atherosclerosis and aneurysm formation in ApoE−/− mice. J. Cardiovasc. Pharmacol. 53, 60–65 (2009).
Nicholls, S. J. et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA 311, 252–262 (2014).
Wilensky, R. L. et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat. Med. 14, 1059–1066 (2008).
Investigators, S. et al. Darapladib for preventing ischemic events in stable coronary heart disease. N. Engl. J. Med. 370, 1702–1711 (2014).
Nash, P., Whitty, A., Handwerker, J., Macen, J. & McFadden, G. Inhibitory specificity of the anti-inflammatory myxoma virus serpin, SERP-1. J. Biol. Chem. 273, 20982–20991 (1998).
Wesley, R. B. 2nd, Meng, X., Godin, D. & Galis, Z. S. Extracellular matrix modulates macrophage functions characteristic to atheroma: collagen type I enhances acquisition of resident macrophage traits by human peripheral blood monocytes in vitro. Arterioscler. Thromb. Vasc. Biol. 18, 432–440 (1998).
Imazio, M. et al. COlchicine for the Prevention of the Post-pericardiotomy Syndrome (COPPS): a multicentre, randomized, double-blind, placebo-controlled trial. Eur. Heart J. 31, 2749–2754 (2010).
Bulgarelli, A., Martins Dias, A. A., Caramelli, B. & Maranhao, R. C. Treatment with methotrexate inhibits atherogenesis in cholesterol-fed rabbits. J. Cardiovasc. Pharmacol. 59, 308–314 (2012).
Everett, B. M. et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am. Heart J. 166, 199–207.e15 (2013).
Acknowledgements
The authors acknowledge funding from the British Heart Foundation Oxford Centre for Research Excellence (N.R., R.P.C.), Oxford NIHR Biomedical Research Centre (R.P.C.), and US National Institutes of Health (E.A.F.).
Author information
Authors and Affiliations
Contributions
All the authors researched data for the article, discussed its contents, and wrote, reviewed, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ruparelia, N., Chai, J., Fisher, E. et al. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol 14, 133–144 (2017). https://doi.org/10.1038/nrcardio.2016.185
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2016.185
This article is cited by
-
S100A8/A9 as a prognostic biomarker with causal effects for post-acute myocardial infarction heart failure
Nature Communications (2024)
-
Could Pan-Immune-Inflammation Value be a Marker for the Diagnosis of Coronary Slow Flow Phenomenon?
Cardiovascular Toxicology (2024)
-
Protective effect of canagliflozin on post-resuscitation myocardial function in a rat model of cardiac arrest
Intensive Care Medicine Experimental (2023)
-
Occupational quartz and particle exposure affect systemic levels of inflammatory markers related to inflammasome activation and cardiovascular disease
Environmental Health (2023)
-
Sex differences in Black Veterans with PTSD: women versus men have higher sympathetic activity, inflammation, and blunted cardiovagal baroreflex sensitivity
Clinical Autonomic Research (2023)