Key Points
-
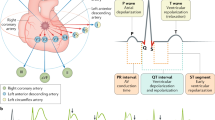
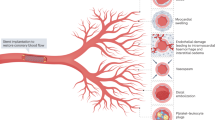
Intramyocardial haemorrhage arises owing to impairment of microvascular function and myocardial perfusion after reperfusion therapy, despite restoration of epicardial vessel patency
-
This major adverse event is associated with large infarct size, adverse left ventricular remodelling, major adverse cardiac events, and death
-
Although intramyocardial haemorrhage has been recognized since the early 1960s, the pathophysiology remains largely unclear
-
During thrombotic occlusion of a coronary artery, cleavage of adherence and tight-junction proteins in the ischaemic endothelium occurs, which leads to extravasation of erythrocytes into the myocardium upon reperfusion
-
Potential therapeutic strategies for the prevention or attenuation of intramyocardial haemorrhage will include protection of the microvasculature or adjustments to reperfusion
Abstract
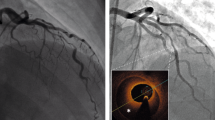
In patients with acute myocardial infarction (AMI), the guideline-recommended treatment is mechanical revascularization by percutaneous coronary intervention (PCI), which is effective at reducing mortality. However, a substantial proportion of patients with AMI develop chronic cardiac failure owing to poor restoration of microvascular function and myocardial perfusion, despite restoration of epicardial vessel patency. This occurrence is called the 'no-reflow' phenomenon. Although pathological and clinical observations initially seemed to support the hypothesis that no-reflow was the result of microvascular obstruction, irreversible microvascular injury and subsequent intramyocardial haemorrhage are now also thought to be important factors in this process. Intramyocardial haemorrhage shares several pathophysiological features with the haemorrhagic transformation that occurs after ischaemic stroke. Understanding of the role of intramyocardial haemorrhage in the no-reflow phenomenon and myocardial injury is crucial to the development of new therapeutic strategies to treat AMI. In this Review, we provide a comprehensive overview of the pathogenesis and clinical relevance of intramyocardial haemorrhage, and discuss diagnostic options and future therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Levi, F., Lucchini, F., Negri, E. & La Vecchia, C. Trends in mortality from cardiovascular and cerebrovascular diseases in Europe and other areas of the world. Heart 88, 119–124 (2002).
Rezkalla, S. H., Dharmashankar, K. C., Abdalrahman, I. B. & Kloner, R. A. No-reflow phenomenon following percutaneous coronary intervention for acute myocardial infarction: incidence, outcome, and effect of pharmacologic therapy. J. Interv. Cardiol. 23, 429–436 (2010).
Kloner, R. A., Ganote, C. E. & Jennings, R. B. The no-reflow phenomenon after temporary coronary occlusion in the dog. J. Clin. Invest. 54, 1496–1508 (1974).
Haeck, J. D. et al. Randomized comparison of primary percutaneous coronary intervention with combined proximal embolic protection and thrombus aspiration versus primary percutaneous coronary intervention alone in ST-segment elevation myocardial infarction: the PREPARE (PRoximal Embolic Protection in Acute myocardial infarction and Resolution of ST-Elevation) study. JACC Cardiovasc. Interv. 2, 934–943 (2009).
Mongeon, F. P., Bélisle, P., Joseph, L., Eisenberg, M. J. & Rinfret, S. Adjunctive thrombectomy for acute myocardial infarction: a bayesian meta-analysis. Circ. Cardiovasc. Interv. 3, 6–16 (2010).
Wu, X. et al. The relationship between attenuated plaque identified by intravascular ultrasound and no-reflow after stenting in acute myocardial infarction: the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc. Interv. 4, 495–502 (2011).
Limbruno, U. et al. Distal embolization during primary angioplasty: histopathologic features and predictability. Am. Heart J. 150, 102–108 (2005).
Falk, E. Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death. Autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation 71, 699–708 (1985).
Sakuma, T., Leong-Poi, H., Fisher, N. G., Goodman, N. C. & Kaul, S. Further insights into the no-reflow phenomenon after primary angioplasty in acute myocardial infarction: the role of microthromboemboli. J. Am. Soc. Echocardiogr. 16, 15–21 (2003).
Wu, K. C. CMR of microvascular obstruction and hemorrhage in myocardial infarction. J. Cardiovasc. Magn. Res. 14, 68 (2012).
Amabile, N. et al. Incidence, predictors, and prognostic value of intramyocardial hemorrhage lesions in ST elevation myocardial infarction. Catheter. Cardiovasc. Interv. 79, 1101–1108 (2012).
Robbers, L. F. et al. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur. Heart J. 34, 2346–2353 (2013).
Ganame, J. et al. Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur. Heart J. 30, 1440–1449 (2009).
Husser, O. et al. Cardiovascular magnetic resonance-derived intramyocardial hemorrhage after STEMI: Influence on long-term prognosis, adverse left ventricular remodeling and relationship with microvascular obstruction. Int. J. Cardiol. 167, 2047–2054 (2012).
Ochiai, K. et al. Hemorrhagic myocardial infarction after coronary reperfusion detected in vivo by magnetic resonance imaging in humans: prevalence and clinical implications. J. Cardiovasc. Magn. Reson. 1, 247–256 (1999).
Asanuma, T. et al. Relationship between progressive microvascular damage and intramyocardial hemorrhage in patients with reperfused anterior myocardial infarction: myocardial contrast echocardiographic study. Circulation 96, 448–453 (1997).
Mather, A. N., Fairbairn, T. A., Ball, S. G., Greenwood, J. P. & Plein, S. Reperfusion haemorrhage as determined by cardiovascular MRI is a predictor of adverse left ventricular remodelling and markers of late arrhythmic risk. Heart 97, 453–459 (2011).
Cokic, I. et al. Iron deposition following chronic myocardial infarction as a substrate for cardiac electrical anomalies: initial findings in a canine model. PLoS ONE 8, e73193 (2013).
Goldfarb, J. W., Hasan, U., Zhao, W. & Han, J. Magnetic resonance susceptibility weighted phase imaging for the assessment of reperfusion intramyocardial hemorrhage. Magn. Reson. Med. 71, 1210–1220 (2014).
Kali, A. et al. Chronic manifestation of postreperfusion intramyocardial hemorrhage as regional iron deposition: a cardiovascular magnetic resonance study with ex vivo validation. Circ. Cardiovasc. Imaging 6, 218–228 (2013).
Vahanian, A. Thrombolytic therapy in Europe: current status. Eur. Heart J. 17 (Suppl. E), 21–27 (1996).
Mathey, D. G. et al. Improved survival up to four years after early coronary thrombolysis. Am. J. Cardiol. 61, 524–529 (1988).
Deloche, A. et al. Effect of coronary-artery reperfusion on extent of myocardial-infarction. Am. Heart J. 93, 358–366 (1977).
Jennings, R. B., Sommers, H. M., Smyth, G. A., Flack, H. A. & Linn., N. H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch. Pathol. 70, 68–78 (1960).
Braunwald, E. & Kloner, R. A. Myocardial reperfusion: a double-edged sword? J. Clin. Invest. 76, 1713–1719 (1985).
Maxwell, L. & Gavin, J. B. The role of post-ischaemic reperfusion in the development of microvascular incompetence and ultrastructural damage in the myocardium. Basic Res. Cardiol. 86, 544–553 (1991).
Nevalainen, T. J., Armiger, L. C. & Gavin, J. B. Effects of ischaemia on vasculature. J. Mol. Cell. Cardiol. 18 (Suppl. 4), 7–10 (1986).
Bresnahan, G. F., Roberts, R., Shell, W. E., Ross, J. Jr & Sobel, B. E. Deleterious effects due to hemorrhage after myocardial reperfusion. Am. J. Cardiol. 33, 82–86 (1974).
Kloner, R. A. et al. Influx of neutrophils into the walls of large epicardial coronary arteries in response to ischemia/reperfusion. Circulation 84, 1758–1772 (1991).
Ambrosio, G., Weisman, H. F., Mannisi, J. A. & Becker, L. C. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation 80, 1846–1861 (1989).
Tsao, P. S., Aoki, N., Lefer, D. J., Johnson, G. III & Lefer, A. M. Time course of endothelial dysfunction and myocardial injury during myocardial ischemia and reperfusion in the cat. Circulation 82, 1402–1412 (1990).
Zaman, A. K., French, C. J., Spees, J. L., Binbrek, A. S. & Sobel, B. E. Vascular rhexis in mice subjected to non-sustained myocardial ischemia and its therapeutic implications. Exp. Biol. Med. (Maywood) 236, 598–603 (2011).
French, C. J., Zaman, A. K. M. T., Kelm, R. J. Jr Spees, J. L. & Sobel, B. E. Vascular rhexis: loss of integrity of coronary vasculature in mice subjected to myocardial infarction. Exp. Biol. Med. (Maywood) 235, 966–973 (2010).
Goddard, L. M. & Iruela-Arispe, M. L. Cellular and molecular regulation of vascular permeability. Thromb. Haemost. 109, 407–415 (2013).
Frohlich, G. M., Meier, P., White, S. K., Yellon, D. M. & Hausenloy, D. J. Myocardial reperfusion injury: looking beyond primary PCI. Eur. Heart J. 34, 1714–1722 (2013).
Augustin, H. G., Koh, G. Y., Thurston, G. & Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 10, 165–177 (2009).
Benest, A. V. et al. Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS ONE 8, e70459 (2013).
Fiedler, U. et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103, 4150–4156 (2004).
Felcht, M. et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J. Clin. Invest. 122, 1991–2005 (2012).
Galaup, A. et al. Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation 125, 140–149 (2012).
Bouleti, C. et al. Protective effects of angiopoietin-like 4 on cerebrovascular and functional damages in ischaemic stroke. Eur. Heart J. 34, 3657–3668 (2013).
Roy, D. et al. Role of reactive oxygen species on the formation of the novel diagnostic marker ischaemia modified albumin. Heart 92, 113–114 (2006).
Rodrigo, R., Libuy, M., Feliú, F. & Hasson, D. Oxidative stress-related biomarkers in essential hypertension and ischemia-reperfusion myocardial damage. Dis. Markers 35, 773–790 (2013).
Eitel, I. et al. Endothelin-1 release in acute myocardial infarction as a predictor of long-term prognosis and no-reflow assessed by contrast-enhanced magnetic resonance imaging. Am. Heart J. 159, 882–890 (2010).
Kurnik, P. B., Courtois, M. R. & Ludbrook, P. A. Diastolic stiffening induced by acute myocardial infarction is reduced by early reperfusion. J. Am. Coll. Cardiol. 12, 1029–1036 (1988).
Ghugre, N. R., Pop, M., Barry, J., Connelly, K. A. & Wright, G. A. Quantitative magnetic resonance imaging can distinguish remodeling mechanisms after acute myocardial infarction based on the severity of ischemic insult. Magn. Reson. Med. 70, 1095–1105 (2013).
Garcia-Dorado, D. et al. Determinants of hemorrhagic infarcts. Histologic observations from experiments involving coronary occlusion, coronary reperfusion, and reocclusion. Am. J. Pathol. 137, 301–311 (1990).
Fishbein, M. C. et al. The relationship of vascular injury and myocardial hemorrhage to necrosis after reperfusion. Circulation 62, 1274–1279 (1980).
Willerson, J. T. et al. Abnormal myocardial fluid retention as an early manifestation of ischemic injury. Am. J. Pathol. 87, 159–188 (1977).
Reffelmann, T. & Kloner, R. A. Microvascular reperfusion injury: rapid expansion of anatomic no reflow during reperfusion in the rabbit. Am. J. Physiol. Heart Circ. Physiol. 283, H1099–H1107 (2002).
Higginson, L. A. et al. Determinants of myocardial hemorrhage after coronary reperfusion in the anesthetized dog. Circulation 65, 62–69 (1982).
Basso, C. & Thiene, G. The pathophysiology of myocardial reperfusion: a pathologist's perspective. Heart 92, 1559–1562 (2006).
Olafsson, B. et al. Reduction of reperfusion injury in the canine preparation by intracoronary adenosine: importance of the endothelium and the no-reflow phenomenon. Circulation 76, 1135–1145 (1987).
Kumar, A. et al. Detection and quantification of myocardial reperfusion hemorrhage using T2*-weighted CMR. JACC Cardiovasc. Imaging 4, 1274–1283 (2011).
Marra, M. P. et al. The contribution of intramyocardial hemorrhage to the “no-reflow phenomenon”: a study performed by cardiac magnetic resonance. Echocardiography 27, 1120–1129 (2010).
Pislaru, S. V. et al. Infarct size, myocardial hemorrhage, and recovery of function after mechanical versus pharmacological reperfusion: effects of lytic state and occlusion time. Circulation 96, 659–666 (1997).
Capone, R. J. & Most, A. S. Myocardial hemorrhage after coronary reperfusion in pigs. Am. J. Cardiol. 41, 259–266 (1978).
Mathur, V. S., Guinn, G. A. & Burris, W. H. Maximal revascularization (reperfusion) in intact conscious dogs after 2 to 5 hours of coronary-occlusion. Am. J. Cardiol. 36, 252–261 (1975).
Kloner, R. A., Ganote, C. E., Jennings, R. B. & Reimer, K. A. Demonstration of the “no-reflow” phenomenon in the dog heart after temporary ischemia. Recent Adv. Stud. Cardiac Struct. Metab. 10, 463–474 (1975).
Chappell, D. et al. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc. Res. 83, 388–396 (2009).
Maksimenko, A. V. & Turashev, A. D. No-reflow phenomenon and endothelial glycocalyx of microcirculation. Biochem. Res. Int. 2012, 859231 (2012).
Kloner, R. A. Does reperfusion injury exist in humans. J. Am. Coll. Cardiol. 21, 537–545 (1993).
Kloner, R. A. et al. Ultrastructural evidence of microvascular damage and myocardial cell injury after coronary artery occlusion: which comes first? Circulation 62, 945–952 (1980).
van der Pouw Kraan, T. C. et al. Systemic toll-like receptor and interleukin-18 pathway activation in patients with acute ST elevation myocardial infarction. J. Mol. Cell. Cardiol. 67, 94–102 (2014).
Burger, D. & Touyz, R. M. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells. J. Am. Soc. Hypertens. 6, 85–99 (2012).
Laskowitz, D. T., Kasner, S. E., Saver, J., Remmel, K. S. & Jauch, E. C. Clinical usefulness of a biomarker-based diagnostic test for acute stroke: the Biomarker Rapid Assessment in Ischemic Injury (BRAIN) study. Stroke 40, 77–85 (2009).
Jugdutt, B. I. et al. Aging-related early changes in markers of ventricular and matrix remodeling after reperfused ST-segment elevation myocardial infarction in the canine model: effect of early therapy with an angiotensin II type 1 receptor blocker. Circulation 122, 341–351 (2010).
Dignat-George, F. & Boulanger, C. M. The many faces of endothelial microparticles. Arterioscler. Thromb. Vasc. Biol. 31, 27–33 (2011).
Maroko, P. R. et al. Coronary-artery reperfusion. I. Early effects on local myocardial function and extent of myocardial necrosis. J. Clin. Invest. 51, 2710–2716 (1972).
Ginks, W. R. et al. Coronary-artery reperfusion. II. Reduction of myocardial infarct size at 1 week after coronary occlusion. J. Clin. Invest. 51, 2717–2723 (1972).
Viehman, G. E., Ma, X. L., Lefer, D. J. & Lefer, A. M. Time course of endothelial dysfunction and myocardial injury during coronary arterial occlusion. Am. J. Physiol. 261, H874–H881 (1991).
Lang, T. W. et al. Consequences of reperfusion after coronary-occlusion—effects on hemodynamic and regional myocardial metabolic function. Am. J. Cardiol. 33, 69–81 (1974).
Morishima, I. et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J. Am. Coll. Cardiol. 36, 1202–1209 (2000).
Haeck, J. D. et al. Proximal embolic protection in patients undergoing primary angioplasty for acute myocardial infarction (PREPARE): core lab adjudicated angiographic outcomes of a randomised controlled trial. Neth. Heart J. 18, 531–536 (2010).
Echavarría-Pinto, M. et al. Safety and efficacy of intense antithrombotic treatment and percutaneous coronary intervention deferral in patients with large intracoronary thrombus. Am. J. Cardiol. 111, 1745–1750 (2013).
Sezer, M. et al. Intracoronary streptokinase after primary percutaneous coronary intervention. N. Engl. J. Med. 356, 1823–1834 (2007).
Reimer, K. A., Lowe, J. E., Rasmussen, M. M. & Jennings, R. B. Wavefront phenomenon of ischemic cell-death. 1. Myocardial infarct size vs duration of coronary-occlusion in dogs. Circulation 56, 786–794 (1977).
McNamara, J. J., Lacro, R. V., Yee, M. & Smith, G. T. Hemorrhagic infarction and coronary reperfusion. J. Thorac. Cardiovasc. Surg. 81, 498–501 (1981).
Driesen, R. B. et al. Histological correlate of a cardiac magnetic resonance imaged microvascular obstruction in a porcine model of ischemia-reperfusion. Cardiovasc. Pathol. 21, 129–131 (2012).
Gertz, S. D., Kalan, J. M., Kragel, A. H., Roberts, W. C. & Braunwald, E. Cardiac morphologic findings in patients with acute myocardial infarction treated with recombinant tissue plasminogen activator. Am. J. Cardiol. 65, 953–961 (1990).
Fujiwara, H. et al. A clinicopathologic study of patients with hemorrhagic myocardial infarction treated with selective coronary thrombolysis with urokinase. Circulation 73, 749–757 (1986).
Mathey, D. G., Schofer, J., Kuck, K. H., Beil, U. & Klöppel, G. Transmural, haemorrhagic myocardial infarction after intracoronary streptokinase. Clinical, angiographic, and necropsy findings. Br. Heart J. 48, 546–551 (1982).
Matsuda, M. et al. Quantitative analysis of infarct size, contraction band necrosis, and coagulation necrosis in human autopsied hearts with acute myocardial infarction after treatment with selective intracoronary thrombolysis. Circulation 76, 981–989 (1987).
Wu, D. J. et al. Clinicopathological study of myocardial infarction with normal or nearly normal extracardiac coronary arteries. Quantitative analysis of contraction band necrosis, coagulation necrosis, hemorrhage, and infarct size. Heart Vessels 6, 55–62 (1990).
Waller, B. F. et al. Status of the myocardium and infarct-related coronary artery in 19 necropsy patients with acute recanalization using pharmacologic (streptokinase, r-tissue plasminogen activator), mechanical (percutaneous transluminal coronary angioplasty) or combined types of reperfusion therapy. J. Am. Coll. Cardiol. 9, 785–801 (1987).
Topol, E. J., Herskowitz, A. & Hutchins, G. M. Massive hemorrhagic myocardial-infarction after coronary thrombolysis. Am. J. Med. 81, 339–343 (1986).
Kali, A., Tang, R. L., Kumar, A., Min, J. K. & Dharmakumar, R. Detection of acute reperfusion myocardial hemorrhage with cardiac mr imaging: T2 versus T2*. Radiology 269, 387–395 (2013).
Kidambi, A. et al. The effect of microvascular obstruction and intramyocardial hemorrhage on contractile recovery in reperfused myocardial infarction: insights from cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 15, 58 (2013).
Beek, A. M., Nijveldt, R. & van Rossum, A. C. Intramyocardial hemorrhage and microvascular obstruction after primary percutaneous coronary intervention. Int. J. Cardiovasc. Imaging 26, 49–55 (2010).
Nordmann, A. J., Bucher, H., Hengstler, P., Harr, T. & Young, J. Primary stenting versus primary balloon angioplasty for treating acute myocardial infarction. Cochrane Database of Systematic Reviews, Issue 2. Art. No.: CD005313. http://dx.doi.org/10.1002/14651858.CD005313.
Ito, H. et al. Clinical implications of the 'no reflow' phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 93, 223–228 (1996).
Ndrepepa, G. et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 55, 2383–2389 (2010).
Payne, A. R. et al. Bright-blood T2-weighted MRI has higher diagnostic accuracy than dark-blood short tau inversion recovery MRI for detection of acute myocardial infarction and for assessment of the ischemic area at risk and myocardial salvage. Circ. Cardiovasc. Imaging 4, 210–219 (2011).
Choi, S. H. et al. Investigation of T2-weighted signal intensity of infarcted myocardium and its correlation with delayed enhancement magnetic resonance imaging in a porcine model with reperfused acute myocardial infarction. Int. J. Cardiovasc. Imaging 25 (Suppl. 1), 111–119 (2009).
Pedersen, S. F. et al. Assessment of intramyocardial hemorrhage by T1-weighted cardiovascular magnetic resonance in reperfused acute myocardial infarction. J. Cardiovasc. Magn. Reson. 14, 59 (2012).
O'Regan, D. P. et al. Assessment of severe reperfusion injury with T2* cardiac MRI in patients with acute myocardial infarction. Heart 96, 1885–1891 (2010).
Bradley, W. G. Jr MR appearance of hemorrhage in the brain. Radiology 189, 15–26 (1993).
Basso, C. et al. Morphologic validation of reperfused hemorrhagic myocardial infarction by cardiovascular magnetic resonance. Am. J. Cardiol. 100, 1322–1327 (2007).
Bekkers, S. C. et al. Clinical implications of microvascular obstruction and intramyocardial haemorrhage in acute myocardial infarction using cardiovascular magnetic resonance imaging. Eur. Radiol. 20, 2572–2578 (2010).
Eitel, I. et al. Prognostic value and determinants of a hypointense infarct core in T2-weighted cardiac magnetic resonance in acute reperfused ST-elevation-myocardial infarction. Circ. Cardiovasc. Imaging 4, 354–362 (2011).
Weaver, J. C. et al. Dynamic changes in ST segment resolution after myocardial infarction and the association with microvascular injury on cardiac magnetic resonance imaging. Heart Lung Circ. 20, 111–118 (2011).
Malek, L. A. et al. Platelet reactivity and intramyocardial hemorrhage in patients with ST-segment elevation myocardial infarction. Clin. Appl. Thromb. Hemost. 20, 553–558 (2013).
Tritto, I., Zuchi, C., Vitale, S. & Ambrosio, G. Therapy against reperfusion-induced microvascular injury. Curr. Pharm. Des. 19, 4586–4596 (2013).
Foltz, W. D. et al. MRI relaxation fluctuations in acute reperfused hemorrhagic infarction. Magn. Reson. Med. 56, 1311–1319 (2006).
White, S. K., Hausenloy, D. J. & Moon, J. C. Imaging the myocardial microcirculation post-myocardial infarction. Curr. Heart Fail. Rep. 9, 282–292 (2012).
O'Regan, D. P. et al. Reperfusion hemorrhage following acute myocardial infarction: assessment with T2* mapping and effect on measuring the area at risk. Radiology 250, 916–922 (2009).
Bekkers, S. C. et al. Detection and characteristics of microvascular obstruction in reperfused acute myocardial infarction using an optimized protocol for contrast-enhanced cardiovascular magnetic resonance imaging. Eur. Radiol. 19, 2904–2912 (2009).
Bogaert, J., Kalantzi, M., Rademakers, F. E., Dymarkowski, S. & Janssens, S. Determinants and impact of microvascular obstruction in successfully reperfused ST-segment elevation myocardial infarction. Assessment by magnetic resonance imaging. Eur. Radiol. 17, 2572–2580 (2007).
Ye, Y. X. et al. Monitoring of monocyte recruitment in reperfused myocardial infarction with intramyocardial hemorrhage and microvascular obstruction by combined fluorine 19 and proton cardiac magnetic resonance imaging. Circulation 128, 1878–1888 (2013).
Wolfe, C. L. et al. Assessment of myocardial salvage after ischemia and reperfusion using magnetic resonance imaging and spectroscopy. Circulation 80, 969–982 (1989).
Ikeno, F., Inagaki, K., Rezaee, M. & Mochly-Rosen, D. Impaired perfusion after myocardial infarction is due to reperfusion-induced δPKC-mediated myocardial damage. Cardiovasc. Res. 73, 699–709 (2007).
Higginson, L. A., Beanlands, D. S., Nair, R. C., Temple, V. & Sheldrick, K. The time course and characterization of myocardial hemorrhage after coronary reperfusion in the anesthetized dog. Circulation 67, 1024–1031 (1983).
Reffelmann, T., Hale, S. L., Li, G. & Kloner, R. A. Relationship between no reflow and infarct size as influenced by the duration of ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 282, H766–H772 (2002).
van der Hoeven, N. W. et al. Clinical parameters associated with collateral development in patients with chronic total coronary occlusion. Heart 99, 1100–1105 (2013).
Teunissen, P. F., Horrevoets, A. J. & van Royen, N. The coronary collateral circulation: genetic and environmental determinants in experimental models and humans. J. Mol. Cell. Cardiol. 52, 897–904 (2012).
Stork, A. et al. Characterization of the peri-infarction zone using T2-weighted MRI and delayed-enhancement MRI in patients with acute myocardial infarction. Eur. Radiol. 16, 2350–2357 (2006).
Tartan, Z. et al. Metabolic syndrome is a predictor for an ECG sign of no-reflow after primary PCI in patients with acute ST-elevation myocardial infarction. Nutr. Metab. Cardiovasc. Dis. 18, 441–447 (2008).
Copin, J. C. & Gasche, Y. Effect of the duration of middle cerebral artery occlusion on the risk of hemorrhagic transformation after tissue plasminogen activator injection in rats. Brain Res. 1243, 161–166 (2008).
Moulin, T., Crépin-Leblond, T., Chopard, J. L. & Bogousslavsky, J. Hemorrhagic infarcts. Eur. Neurol. 34, 64–77 (1994).
Alexandrov, A. V., Black, S. E., Ehrlich, L. E., Caldwell, C. B. & Norris, J. W. Predictors of hemorrhagic transformation occurring spontaneously and on anticoagulants in patients with acute ischemic stroke. Stroke 28, 1198–1202 (1997).
Molina, C. A. et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke 32, 1079–1084 (2001).
Jickling, G. C. et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J. Cereb. Blood Flow Metab. 34, 185–199 (2014).
Sussman, E. S. & Connolly, E. S. Jr. Hemorrhagic transformation: a review of the rate of hemorrhage in the major clinical trials of acute ischemic stroke. Front. Neurol. 4, 69 (2013).
Jauch, E. C. et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44, 870–947 (2013).
Arboix, A. & Alio, J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr. Cardiol. Rev. 6, 150–161 (2010).
Ames, A., Wright, R. L., Kowada, M., Thurston, J. M. & Majno, G. Cerebral ischemia. II. The no-reflow phenomenon. Am. J. Pathol. 52, 437–453 (1968).
Heusch, G. Reduction of infarct size by ischaemic post-conditioning in humans: fact or fiction? Eur. Heart J. 33, 13–15 (2012).
Garcia-Dorado, D. et al. Intracoronary infusion of superoxide dismutase and reperfusion injury in the pig heart. Basic Res. Cardiol. 85, 619–629 (1990).
Turer, A. T. & Hill, J. A. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am. J. Cardiol. 106, 360–368 (2010).
Hausenloy, D. J. & Yellon, D. M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 123, 92–100 (2013).
Yellon, D. M. & Hausenloy, D. J. Myocardial reperfusion injury. N. Engl. J. Med. 357, 1121–1135 (2007).
Kloner, R. A. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ. Res. 113, 451–463 (2013).
Zhang, J. et al. Collagen-targeting vascular endothelial growth factor improves cardiac performance after myocardial infarction. Circulation 119, 1776–1784 (2009).
Zan, L. et al. Src regulates angiogenic factors and vascular permeability after focal cerebral ischemia-reperfusion. Neuroscience 262, 118–128 (2014).
Huang, R. L. et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood 118, 3990–4002 (2011).
Sandhu, R. et al. Reciprocal regulation of angiopoietin-1 and angiopoietin-2 following myocardial infarction in the rat. Cardiovasc. Res. 64, 115–124 (2004).
Lee, S. W. et al. Angiopoietin-1 protects heart against ischemia/reperfusion injury through VE-cadherin dephosphorylation and myocardiac integrin-β1/ERK/caspase-9 phosphorylation cascade. Mol. Med. 17, 1095–1106 (2011).
Inzitari, D. et al. MMP9 variation after thrombolysis is associated with hemorrhagic transformation of lesion and death. Stroke 44, 2901–2903 (2013).
Hlatky, M. A. et al. Matrix metalloproteinase circulating levels, genetic polymorphisms, and susceptibility to acute myocardial infarction among patients with coronary artery disease. Am. Heart J. 154, 1043–1051 (2007).
Sumii, T. & Lo, E. H. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke 33, 831–836 (2002).
Simpson, P. J., Fantone, J. C., Mickelson, J. K., Gallagher, K. P. & Lucchesi, B. R. Identification of a time window for therapy to reduce experimental canine myocardial injury—suppression of neutrophil activation during 72 hours of reperfusion. Circ. Res. 63, 1070–1079 (1988).
Litt, M. R., Jeremy, R. W., Weisman, H. F., Winkelstein, J. A. & Becker, L. C. Neutrophil depletion limited to reperfusion reduces myocardial infarct size after 90 minutes of ischemia. Evidence for neutrophil-mediated reperfusion injury. Circulation 80, 1816–1827 (1989).
Romson, J. L. et al. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation 67, 1016–1023 (1983).
Okamura, K., Tsubokawa, T., Johshita, H., Miyazaki, H. & Shiokawa, Y. Edaravone, a free radical scavenger, attenuates cerebral infarction and hemorrhagic infarction in rats with hyperglycemia. Neurol. Res. 36, 65–69 (2014).
Feng, S. et al. Edaravone for acute ischaemic stroke. Cochrane Database of Systematic Reviews. Issue 12. Art. No.: CD007230. http://dx.doi.org/14651858.CD007230.pub2.
Jang, J. W. et al. Melatonin reduced the elevated matrix metalloproteinase-9 level in a rat photothrombotic stroke model. J. Neurol. Sci. 323, 221–227 (2012).
Zhang, L. et al. Atorvastatin extends the therapeutic window for tPA to 6 h after the onset of embolic stroke in rats. J. Cereb. Blood Flow Metab. 29, 1816–1824 (2009).
Machado, L. S. et al. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 7, 56 (2006).
Slavic, S. et al. Cannabinoid receptor 1 inhibition improves cardiac function and remodelling after myocardial infarction and in experimental metabolic syndrome. J. Mol. Med. (Berl.) 91, 811–823 (2013).
Isahaya, K. et al. Effects of edaravone, a free radical scavenger, on serum levels of inflammatory biomarkers in acute brain infarction. J. Stroke Cerebrovasc. Dis. 21, 102–107 (2012).
Copin, J. C., Merlani, P., Sugawara, T., Chan, P. H. & Gasche, Y. Delayed matrix metalloproteinase inhibition reduces intracerebral hemorrhage after embolic stroke in rats. Exp. Neurol. 213, 196–201 (2008).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Lindahl, P., Johansson, B. R., Levéen, P. & Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277, 242–245 (1997).
Hellström, M. et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol. 153, 543–553 (2001).
Hall, C. N. et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 (2014).
Motiejūnaite, R. & Kazlauskas, A. Pericytes and ocular diseases. Exp. Eye Res. 86, 171–177 (2008).
O'Farrell, F. M. & Attwell, D. A role for pericytes in coronary no-reflow. Nat. Rev. Cardiol. 11, 427–432 (2014).
Sato, H., Jordan, J. E., Zhao, Z. Q., Sarvotham, S. S. & Vinten-Johansen, J. Gradual reperfusion reduces infarct size and endothelial injury but augments neutrophil accumulation. Ann. Thorac. Surg. 64, 1099–1107 (1997).
Okamoto, F., Allen, B. S., Buckberg, G. D., Bugyi, H. & Leaf, J. Reperfusion conditions: importance of ensuring gentle versus sudden reperfusion during relief of coronary occlusion. J. Thorac. Cardiovasc. Surg. 92, 613–620 (1986).
Heusch, G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet 381, 166–175 (2013).
Bodi, V. et al. Effect of ischemic postconditioning on microvascular obstruction in reperfused myocardial infarction. Results of a randomized study in patients and of an experimental model in swine. Int. J. Cardiol. 175, 138–146 (2014).
Lindal, S. et al. Endothelial injury and trapping of blood cells in human myocardium following coronary bypass surgery. Scand. Cardiovasc. J. 33, 143–150 (1999).
Peng, C. F. et al. The adverse effect of systemic hypertension following myocardial reperfusion. J. Surg. Res. 34, 59–67 (1983).
Garcia-Dorado, D. et al. Diltiazem and progression of myocardial ischemic damage during coronary artery occlusion and reperfusion in porcine hearts. J. Am. Coll. Cardiol. 10, 906–911 (1987).
Nanas, J. N. et al. Moderate systemic hypotension during reperfusion reduces the coronary blood flow and increases the size of myocardial infarction in pigs. Chest 125, 1492–1499 (2004).
Kunadian, V. et al. Intracoronary pharmacotherapy in the management of coronary microvascular dysfunction. J. Thromb. Thrombolysis 26, 234–242 (2008).
Ibanez, B. et al. The cardioprotection granted by metoprolol is restricted to its administration prior to coronary reperfusion. Int. J. Cardiol. 147, 428–432 (2011).
Pizarro, G. et al. Long term benefit of early pre-reperfusion metoprolol administration in patients with acute myocardial infarction: results from the METOCARD-CNIC trial (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction). J. Am. Coll. Cardiol. 63, 2356–2362 (2014).
Movahed, M. R. & Butman, S. M. The pathogenesis and treatment of no-reflow occurring during percutaneous coronary intervention. Cardiovasc. Revasc. Med. 9, 56–61 (2008).
Wolfe, C. L., Donnelly, T. J., Sievers, R. & Parmley, W. W. Myocardial protection with verapamil during ischemia and reperfusion: dissociation between myocardial salvage and the degree of ATP depletion during ischemia. Cardiovasc. Res. 25, 101–109 (1991).
Rousseau, G. et al. Diltiazem at reperfusion reduces neutrophil accumulation and infarct size in dogs with ischemic myocardium. Cardiovasc. Res. 25, 319–329 (1991).
Di Pasquale, P., Paterna, S., Cannizzaro, S. & Bucca, V. Does captopril treatment before thrombolysis in acute myocardial-infarction attenuate reperfusion damage short-term and long-term effects. Int. J. Cardiol. 43, 43–50 (1994).
O'Gara, P. T. et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127, e362–e425 (2013).
De Luca, G., Navarese, E. & Marino, P. Risk profile and benefits from Gp IIb-IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-regression analysis of randomized trials. Eur. Heart J. 30, 2705–2713 (2009).
Petronio, A. S. et al. Impact of early abciximab administration on infarct size in patients with ST-elevation myocardial infarction. Int. J. Cardiol. 155, 230–235 (2012).
Buszman, P. P. et al. Controlled reperfusion with intravenous bivalirudin and intracoronary abciximab combination therapy in the porcine myocardial infarction model. Thromb. Res. 130, 265–272 (2012).
Rodríguez-González, R. et al. Platelet derived growth factor-CC isoform is associated with hemorrhagic transformation in ischemic stroke patients treated with tissue plasminogen activator. Atherosclerosis 226, 165–171 (2013).
Fredriksson, L., Li, H., Fieber, C., Li, X. & Eriksson, U. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 23, 3793–3802 (2004).
Li, X. et al. PDGF-C is a new protease-activated ligand for the PDGF α-receptor. Nat. Cell Biol. 2, 302–309 (2000).
Su, E. J. et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat. Med. 14, 731–737 (2008).
Author information
Authors and Affiliations
Contributions
R.P.B and G.A.d.W. contributed equally to this article. R.P.B. researched data for the article, made substantial contribution to discussion of the content, and wrote the manuscript. N.v.R. and G.A.d.W. made substantial contribution to discussion of the content, wrote, reviewed, and edited the manuscript before submission. R.N. and A.M.B. made substantial contribution to discussion of the content, reviewed, and edited the manuscript before submission. J.E. wrote, reviewed, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Betgem, R., de Waard, G., Nijveldt, R. et al. Intramyocardial haemorrhage after acute myocardial infarction. Nat Rev Cardiol 12, 156–167 (2015). https://doi.org/10.1038/nrcardio.2014.188
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2014.188
This article is cited by
-
Imatinib attenuates reperfusion injury in a rat model of acute myocardial infarction
Basic Research in Cardiology (2023)
-
Quantitative susceptibility mapping (QSM) of the cardiovascular system: challenges and perspectives
Journal of Cardiovascular Magnetic Resonance (2022)
-
Effect of ticagrelor and prasugrel on remote myocardial inflammation in patients with acute myocardial infarction with ST-elevation: a CMR T1 and T2 mapping study
The International Journal of Cardiovascular Imaging (2022)
-
Assessment of intramyocardial hemorrhage with dark-blood T2*-weighted cardiovascular magnetic resonance
Journal of Cardiovascular Magnetic Resonance (2021)
-
Prognostic value and clinical predictors of intramyocardial hemorrhage measured by CMR T2* sequences in STEMI
The International Journal of Cardiovascular Imaging (2021)