Key Points
-
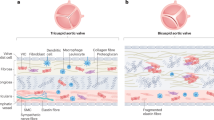
Calcific aortic valve disease (CAVD) seems to be distinct from vascular calcification in terms of both disease mechanisms and progression, although some risk factors are shared
-
Valve-specific drug targets are likely to be required to prevent or treat CAVD, given that drug strategies for vascular disease have not proven successful when applied to CAVD
-
Ubiquitous targeting of the cytokines thought to lead to valve disease is not likely to be feasible or beneficial, because they are required for wound healing elsewhere in the body
-
Targeting specific combinations of G-protein-coupled receptors might simultaneously prevent myofibroblast activation and collagen accumulation, which are thought to be two of the main initiators of, and contributors to, CAVD
-
Functional blocking of cadherin-11 might be an effective target to prevent mechanotransduction between valve cells that is thought to exacerbate cytokine signalling and synergistically lead to calcification
-
Lipid lowering might be an effective strategy for CAVD treatment; however, a targeted approach centred on lowering the lipoprotein(a) level and increasing the HDL-cholesterol level might be required
Abstract
Calcific aortic valve disease (CAVD) is a major contributor to cardiovascular morbidity and mortality and, given its association with age, the prevalence of CAVD is expected to continue to rise as global life expectancy increases. No drug strategies currently exist to prevent or treat CAVD. Given that valve replacement is the only available clinical option, patients often cope with a deteriorating quality of life until diminished valve function demands intervention. The recognition that CAVD results from active cellular mechanisms suggests that the underlying pathways might be targeted to treat the condition. However, no such therapeutic strategy has been successfully developed to date. One hope was that drugs already used to treat vascular complications might also improve CAVD outcomes, but the mechanisms of CAVD progression and the desired therapeutic outcomes are often different from those of vascular diseases. Therefore, we discuss the benchmarks that must be met by a CAVD treatment approach, and highlight advances in the understanding of CAVD mechanisms to identify potential novel therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Go, A. S. et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 127, e6–e245 (2013).
Nkomo, V. T. et al. Burden of valvular heart diseases: a population-based study. Lancet 368, 1005–1011 (2006).
Rajamannan, N. M. et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease—2011 update. Circulation 124, 1783–1791 (2011).
Bach, D. S. Prevalence and characteristics of unoperated patients with severe aortic stenosis. J. Heart Valve Dis. 20, 284–291 (2011).
Lindman, B. R., Bonow, R. O. & Otto, C. M. Current management of calcific aortic stenosis. Circ. Res. 113, 223–237 (2013).
Daneault, B. et al. Stroke associated with surgical and transcatheter treatment of aortic stenosis: a comprehensive review. J. Am. Coll. Cardiol. 58, 2143–2150 (2011).
Sacks, M. S. & Yoganathan, A. P. Heart valve function: a biomechanical perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 1369–1391 (2007).
Sacks, M. S., Smith, D. B. & Hiester, E. D. The aortic valve microstructure: effects of transvalvular pressure. J. Biomed. Mat. Res. 41, 131–141 (1998).
Stella, J. A., Liao, J. & Sacks, M. S. Time-dependent biaxial mechanical behavior of the aortic heart valve leaflet. J. Biomech. 40, 3169–3177 (2007).
Schoen, F. J. Aortic valve structure-function correlations: role of elastic fibers no longer a stretch of the imagination. J. Heart Valve Dis. 6, 1–6 (1997).
Stephens, E. H., Chu, C. K. & Grande-Allen, K. J. Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: relevance to an age-specific tissue-engineered heart valve. Acta Biomater. 4, 1148–1160 (2008).
Otto, C. M., Kuusisto, J., Reichenbach, D. D., Gown, A. M. & O'Brien, K. D. Characterization of the early lesion of 'degenerative' valvular aortic stenosis: histological and immunohistochemical studies. Circulation 90, 844–853 (1994).
Butcher, J. T. et al. Transcriptional profiles of valvular and vascular endothelial cells reveal phenotypic differences: influence of shear stress. Arterioscler. Thromb. Vasc. Biol. 26, 69–77 (2006).
Cucina, A. et al. Shear stress induces changes in the morphology and cytoskeleton organisation of arterial endothelial cells. Eur. J. Vasc. Endovasc. Surg. 9, 86–92 (1995).
Stamatas, G. N. & McIntire, L. V. Rapid flow-induced responses in endothelial cells. Biotechnol. Prog. 17, 383–402 (2001).
Butcher, J. T. & Nerem, R. M. Valvular endothelial cells and the mechanoregulation of valvular pathology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 1445–1457 (2007).
Sucosky, P., Balachandran, K., Elhammali, A., Jo, H. & Yoganathan, A. P. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-β1-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 29, 254–260 (2009).
Balachandran, K. et al. Cyclic strain induces dual-mode endothelial-mesenchymal transformation of the cardiac valve. Proc. Natl Acad. Sci. USA 108, 19943–19948 (2011).
Bischoff, J. & Aikawa, E. Progenitor cells confer plasticity to cardiac valve endothelium. J. Cardiovasc. Transl. Res. 4, 710–719 (2011).
Holliday, C. J., Ankeny, R. F., Jo, H. & Nerem, R. M. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 301, H856–H867 (2011).
Simmons, C. A., Grant, G. R., Manduchi, E. & Davies, P. F. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ. Res. 96, 792–799 (2005).
Gould, S. T., Srigunapalan, S., Simmons, C. A. & Anseth, K. S. Hemodynamic and cellular response feedback in calcific aortic valve disease. Circ. Res. 113, 186–197 (2013).
Ankeny, R. F. et al. Preferential activation of SMAD1/5/8 on the fibrosa endothelium in calcified human aortic valves—association with low BMP antagonists and SMAD6. PLoS ONE 6, e20969 (2011).
Hergenreider, E. et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 14, 249–256 (2012).
Butcher, J. T. & Nerem, R. M. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue Eng. 12, 905–915 (2006).
Tseng, H. et al. A three-dimensional co-culture model of the aortic valve using magnetic levitation. Acta Biomater. 10, 173–182 (2014).
Bosse, K. et al. Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease. J. Mol. Cell. Cardiol. 60, 27–35 (2013).
Rabkin-Aikawa, E., Farber, M., Aikawa, M. & Schoen, F. J. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J. Heart Valve Dis. 13, 841–847 (2004).
Filip, D. A., Radu, A. & Simionescu, M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ. Res. 59, 310–320 (1986).
Chester, A. H. & Taylor, P. M. Molecular and functional characteristics of heart-valve interstitial cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 1437–1443 (2007).
Hinz, B. et al. The myofibroblast: one function, multiple origins. Am. J. Pathol. 170, 1807–1816 (2007).
Mohler, E. R. 3rd et al. Bone formation and inflammation in cardiac valves. Circulation 103, 1522–1528 (2001).
Chen, J. H., Yip, C. Y., Sone, E. D. & Simmons, C. A. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am. J. Pathol. 174, 1109–1119 (2009).
New, S. E. & Aikawa, E. Cardiovascular calcification: an inflammatory disease. Circ. J. 75, 1305–1313 (2011).
Demer, L. L. & Tintut, Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation 117, 2938–2948 (2008).
Freeman, R. V. & Otto, C. M. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 111, 3316–3326 (2005).
Ortlepp, J. R., Schmitz, F., Bozoglu, T., Hanrath, P. & Hoffmann, R. Cardiovascular risk factors in patients with aortic stenosis predict prevalence of coronary artery disease but not of aortic stenosis: an angiographic pair matched case–control study. Heart 89, 1019–1022 (2003).
Weiss, R. M., Miller, J. D. & Heistad, D. D. Fibrocalcific aortic valve disease: opportunity to understand disease mechanisms using mouse models. Circ. Res. 113, 209–222 (2013).
Miller, J. D., Weiss, R. M. & Heistad, D. D. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ. Res. 108, 1392–1412 (2011).
Adiguzel, E., Ahmad, P. J., Franco, C. & Bendeck, M. P. Collagens in the progression and complications of atherosclerosis. Vasc. Med. 14, 73–89 (2009).
Libby, P. The interface of atherosclerosis and thrombosis: basic mechanisms. Vasc. Med. 3, 225–229 (1998).
Newby, A. C. & Zaltsman, A. B. Fibrous cap formation or destruction—the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc. Res. 41, 345–360 (1999).
Libby, P. Collagenases and cracks in the plaque. J. Clin. Invest. 123, 3201–3203 (2013).
Libby, P. & Sasiela, W. Plaque stabilization: can we turn theory into evidence? Am. J. Cardiol. 98, 26P–33P (2006).
Lin, T. C. et al. Mechanical response of a calcified plaque model to fluid shear force. Ann. Biomed. Eng. 34, 1535–1541 (2006).
Saremi, A., Bahn, G. & Reaven, P. D. Progression of vascular calcification is increased with statin use in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 35, 2390–2392 (2012).
Houslay, E. S. et al. Progressive coronary calcification despite intensive lipid-lowering treatment: a randomised controlled trial. Heart 92, 1207–1212 (2006).
Hjortnaes, J. et al. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: a role for inflammation. Eur. Heart J. 31, 1975–1984 (2010).
Dweck, M. R. et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 125, 76–86 (2012).
Dweck, M. R. et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J. Am. Coll. Cardiol. 59, 1539–1548 (2012).
Giachelli, C. M. Vascular calcification mechanisms. J. Am. Soc. Nephrol. 15, 2959–2964 (2004).
Goodman, W. G. et al. Vascular calcification in chronic kidney disease. Am. J. Kidney Dis. 43, 572–579 (2004).
Aikawa, E. et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 116, 2841–2850 (2007).
Aikawa, E. et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation 115, 377–386 (2007).
Carter, S. et al. Sirt1 inhibits resistin expression in aortic stenosis. PLoS ONE 7, e35110 (2012).
Mohty, D. et al. Age-related differences in the pathogenesis of calcific aortic stenosis: the potential role of resistin. Int. J. Cardiol. 142, 126–132 (2010).
New, S. E. et al. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ. Res. 113, 72–77 (2013).
Rajamannan, N. M. et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 107, 2181–2184 (2003).
Wallin, R., Wajih, N., Greenwood, G. T. & Sane, D. C. Arterial calcification: a review of mechanisms, animal models, and the prospects for therapy. Med. Res. Rev. 21, 274–301 (2001).
Neven, E., De Schutter, T. M., De Broe, M. E. & D'Haese, P. C. Cell biological and physicochemical aspects of arterial calcification. Kidney Int. 79, 1166–1177 (2011).
Li, X., Yang, H. Y. & Giachelli, C. M. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis 199, 271–277 (2008).
Dalfino, G. et al. Bone morphogenetic protein-2 may represent the molecular link between oxidative stress and vascular stiffness in chronic kidney disease. Atherosclerosis 211, 418–423 (2010).
Balderman, J. A. et al. Bone morphogenetic protein-2 decreases microRNA-30b and microRNA-30c to promote vascular smooth muscle cell calcification. J. Am. Heart Assoc. 1, e003905 (2012).
Bertazzo, S. et al. Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nat. Mater. 12, 576–583 (2013).
Kapustin, A. N. et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ. Res. 109, e1–e12 (2011).
Ferdous, Z., Jo, H. & Nerem, R. M. Differences in valvular and vascular cell responses to strain in osteogenic media. Biomaterials 32, 2885–2893 (2011).
Yip, C. Y., Chen, J. H., Zhao, R. & Simmons, C. A. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler. Thromb. Vasc. Biol. 29, 936–942 (2009).
O'Brien, K. D. et al. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of 'degenerative' valvular aortic stenosis. Arterioscler. Thromb. Vasc. Biol. 16, 523–532 (1996).
Mohty, D. et al. Association between plasma LDL particle size, valvular accumulation of oxidized LDL, and inflammation in patients with aortic stenosis. Arterioscler. Thromb. Vasc. Biol. 28, 187–193 (2008).
Mahmut, A. et al. Lipoprotein lipase in aortic valve stenosis is associated with lipid retention and remodelling. Eur. J. Clin. Invest. 43, 570–578 (2013).
Nadlonek, N. A., Lee, J. H., Weyant, M. J., Meng, X. & Fullerton, D. A. ox-LDL induces PiT-1 expression in human aortic valve interstitial cells. J. Surg. Res. 184, 6–9 (2013).
Pohle, K. et al. Progression of aortic valve calcification: association with coronary atherosclerosis and cardiovascular risk factors. Circulation 104, 1927–1932 (2001).
Aronow, W. S., Ahn, C., Kronzon, I. & Goldman, M. E. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am. J. Cardiol. 88, 693–695 (2001).
Moura, L. M. et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J. Am. Coll. Cardiol. 49, 554–561 (2007).
Novaro, G. M. et al. Effect of hydroxymethylglutaryl coenzyme A reductase inhibitors on the progression of calcific aortic stenosis. Circulation 104, 2205–2209 (2001).
Teo, K. K., Corsi, D. J., Tam, J. W., Dumesnil, J. G. & Chan, K. L. Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebo-controlled clinical trials on 2344 patients. Can. J. Cardiol. 27, 800–808 (2011).
Gould, A. L., Rossouw, J. E., Santanello, N. C., Heyse, J. F. & Furberg, C. D. Cholesterol reduction yields clinical benefit: impact of statin trials. Circulation 97, 946–952 (1998).
Ray, K. K. et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch. Intern. Med. 170, 1024–1031 (2010).
Libby, P. & Aikawa, M. Mechanisms of plaque stabilization with statins. Am. J. Cardiol. 91, 4B–8B (2003).
Libby, P. & Aikawa, M. Effects of statins in reducing thrombotic risk and modulating plaque vulnerability. Clin. Cardiol. 26 (Suppl. 1), I11–I14 (2003).
Benton, J. A., Kern, H. B., Leinwand, L. A., Mariner, P. D. & Anseth, K. S. Statins block calcific nodule formation of valvular interstitial cells by inhibiting alpha-smooth muscle actin expression. Arterioscler. Thromb. Vasc. Biol. 29, 1950–1957 (2009).
Bellamy, M. F., Pellikka, P. A., Klarich, K. W., Tajik, A. J. & Enriquez-Sarano, M. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J. Am. Coll. Cardiol. 40, 1723–1730 (2002).
Cowell, S. J. et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 352, 2389–2397 (2005).
Rossebo, A. B. et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 359, 1343–1356 (2008).
Gerdts, E. et al. Impact of baseline severity of aortic valve stenosis on effect of intensive lipid lowering therapy (from the SEAS study). Am. J. Cardiol. 106, 1634–1639 (2010).
Chan, K. L., Teo, K., Dumesnil, J. G., Ni, A. & Tam, J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 121, 306–314 (2010).
Jassal, D. S. et al. Evaluating the effectiveness of rosuvastatin in preventing the progression of diastolic dysfunction in aortic stenosis: a substudy of the aortic stenosis progression observation measuring effects of rosuvastatin (ASTRONOMER) study. Cardiovasc. Ultrasound 9, 5 (2011).
Stone, N. J. et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. http://dx.doi.org/10.1016/j.jacc.2013.11.002.
Monzack, E. L. & Masters, K. S. A time course investigation of the statin paradox among valvular interstitial cell phenotypes. Am. J. Physiol. Heart Circ. Physiol. 303, H903–H909 (2012).
Capoulade, R. et al. Impact of metabolic syndrome on progression of aortic stenosis: influence of age and statin therapy. J. Am. Coll. Cardiol. 60, 216–223 (2012).
Thanassoulis, G. et al. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 368, 503–512 (2013).
Jacobson, T. A. Lipoprotein(a), cardiovascular disease, and contemporary management. Mayo Clin. Proc. 88, 1294–1311 (2013).
Chu, Y. et al. Pioglitazone attenuates valvular calcification induced by hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 33, 523–532 (2013).
Yontar, O. C. & Yilmaz, M. B. Importance of serum high-density lipoprotein levels to aortic valvular disease. Br. J. Pharmacol. 155, 1163 (2008).
Speidl, W. S. et al. Recombinant apolipoprotein A-I Milano rapidly reverses aortic valve stenosis and decreases leaflet inflammation in an experimental rabbit model. Eur. Heart J. 31, 2049–2057 (2010).
Trapeaux, J. et al. Improvement of aortic valve stenosis by ApoA-I mimetic therapy is associated with decreased aortic root and valve remodelling in mice. Br. J. Pharmacol. 169, 1587–1599 (2013).
Lommi, J. I. et al. High-density lipoproteins (HDL) are present in stenotic aortic valves and may interfere with the mechanisms of valvular calcification. Atherosclerosis 219, 538–544 (2011).
Towler, D. A. Molecular and cellular aspects of calcific aortic valve disease. Circ. Res. 113, 198–208 (2013).
Grinnell, F. & Ho, C. H. Transforming growth factor β stimulates fibroblast-collagen matrix contraction by different mechanisms in mechanically loaded and unloaded matrices. Exp. Cell Res. 273, 248–255 (2002).
Merryman, W. D. et al. Synergistic effects of cyclic tension and transforming growth factor-β1 on the aortic valve myofibroblast. Cardiovasc. Pathol. 16, 268–276 (2007).
Walker, G. A., Masters, K. S., Shah, D. N., Anseth, K. S. & Leinwand, L. A. Valvular myofibroblast activation by transforming growth factor-β: implications for pathological extracellular matrix remodeling in heart valve disease. Circ. Res. 95, 253–260 (2004).
Ghosh, J. et al. The role of transforming growth factor β1 in the vascular system. Cardiovasc. Pathol. 14, 28–36 (2005).
Jian, B., Narula, N., Li, Q. Y., Mohler, E. R. 3rd & Levy, R. J. Progression of aortic valve stenosis: TGF-β1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann. Thorac. Surg. 75, 457–465 (2003).
Fisher, C. I., Chen, J. & Merryman, W. D. Calcific nodule morphogenesis by heart valve interstitial cells is strain dependent. Biomech. Model Mechanobiol. 12, 5–17 (2013).
Chen, J. H., Chen, W. L., Sider, K. L., Yip, C. Y. & Simmons, C. A. β-Catenin mediates mechanically regulated, transforming growth factor-β1-induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler. Thromb. Vasc. Biol. 31, 590–597 (2011).
Howe, P. H. in The Cytokine Handbook (eds Thomson, A. W. & Lotze, M. T.) 1119–1141 (Academic Press Inc., 2003).
Zhang, Y. E. Non-Smad pathways in TGF-β signaling. Cell Res. 19, 128–139 (2009).
Galliher, A. J. & Schiemann, W. P. β3 Integrin and Src facilitate transforming growth factor-β mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 8, R42 (2006).
Hutcheson, J. D., Ryzhova, L. M., Setola, V. & Merryman, W. D. 5-HT2B Antagonism arrests non-canonical TGF-β1-induced valvular myofibroblast differentiation. J. Mol. Cell. Cardiol. 53, 707–714 (2012).
Yanagawa, B. et al. miRNA-141 is a novel regulator of BMP-2-mediated calcification in aortic stenosis. J. Thorac. Cardiovasc. Surg. 144, 256–262 (2012).
Kaden, J. J. et al. Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J. Heart Valve Dis. 13, 560–566 (2004).
Yang, X. et al. Bone morphogenic protein 2 induces Runx2 and osteopontin expression in human aortic valve interstitial cells: role of Smad1 and extracellular signal-regulated kinase 1/2. J. Thorac. Cardiovasc. Surg. 138, 1008–1015 (2009).
Balachandran, K., Sucosky, P., Jo, H. & Yoganathan, A. P. Elevated cyclic stretch induces aortic valve calcification in a bone morphogenic protein-dependent manner. Am. J. Pathol. 177, 49–57 (2010).
Kroeze, W. K., Sheffler, D. J. & Roth, B. L. G-protein-coupled receptors at a glance. J. Cell Sci. 116, 4867–4869 (2003).
Hutcheson, J. D., Setola, V., Roth, B. L. & Merryman, W. D. Serotonin receptors and heart valve disease—it was meant 2B. Pharmacol. Ther. 132, 146–157 (2011).
Gustafsson, B. I., Hauso, O., Drozdov, I., Kidd, M. & Modlin, I. M. Carcinoid heart disease. Int. J. Cardiol. 129, 318–324 (2008).
Connolly, H. M. et al. Valvular heart disease associated with fenfluramine-phentermine. N. Engl. J. Med. 337, 581–588 (1997).
Fitzgerald, L. W. et al. Possible role of valvular serotonin 5-HT2B receptors in the cardiopathy associated with fenfluramine. Mol. Pharmacol. 57, 75–81 (2000).
Rothman, R. B. et al. Evidence for possible involvement of 5-HT2B receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 102, 2836–2841 (2000).
Huang, X. P. et al. Parallel functional activity profiling reveals valvulopathogens are potent 5-hydroxytryptamine2B receptor agonists: implications for drug safety assessment. Mol. Pharmacol. 76, 710–722 (2009).
Setola, V. et al. 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol. Pharmacol. 63, 1223–1229 (2003).
Launay, J. M. et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat. Med. 8, 1129–1135 (2002).
Jaffré, F. et al. Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy. Circ. Res. 104, 113–123 (2009).
Helske, S. et al. Induction of local angiotensin II-producing systems in stenotic aortic valves. J. Am. Coll. Cardiol. 44, 1859–1866 (2004).
Brooke, B. S. et al. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N. Engl. J. Med. 358, 2787–2795 (2008).
Cohn, R. D. et al. Angiotensin II type 1 receptor blockade attenuates TGF-β-induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 13, 204–210 (2007).
Habashi, J. P. et al. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 332, 361–365 (2011).
Olsen, M. H. et al. Effect of losartan versus atenolol on aortic valve sclerosis (a LIFE substudy). Am. J. Cardiol. 94, 1076–1080 (2004).
Côté, N., Couture, C., Pibarot, P., Després, J. -P. & Mathieu, P. Angiotensin receptor blockers are associated with a lower remodelling score of stenotic aortic valves. Eur. J. Clin. Inves. 41, 1172–1179 (2011).
Nadir, M. A. et al. Impact of renin–angiotensin system blockade therapy on outcome in aortic stenosis. J. Am. Coll. Cardiol. 58, 570–576 (2011).
Capoulade, R. et al. Impact of hypertension and renin-angiotensin system inhibitors in aortic stenosis. Eur. J. Clin. Inves. 43, 1262–1272 (2013).
Arishiro, K. et al. Angiotensin receptor-1 blocker inhibits atherosclerotic changes and endothelial disruption of the aortic valve in hypercholesterolemic rabbits. J. Am. Coll. Cardiol. 49, 1482–1489 (2007).
Judge, D. P., Rouf, R., Habashi, J. & Dietz, H. C. Mitral valve disease in Marfan syndrome and related disorders. J. Cardiovasc. Transl. Res. 4, 741–747 (2011).
Chang, S. K. et al. Cadherin-11 regulates fibroblast inflammation. Proc. Natl Acad. Sci. USA 108, 8402–8407 (2011).
Schneider, D. J. et al. Cadherin-11 contributes to pulmonary fibrosis: potential role in TGF-β production and epithelial to mesenchymal transition. FASEB J. 26, 503–512 (2012).
Lee, D. M. et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science 315, 1006–1010 (2007).
Okazaki, M. et al. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J. Biol. Chem. 269, 12092–12098 (1994).
Hinz, B., Pittet, P., Smith-Clerc, J., Chaponnier, C. & Meister, J. J. Myofibroblast development is characterized by specific cell–cell adherens junctions. Mol. Biol. Cell 15, 4310–4320 (2004).
Hutcheson, J. D. et al. Cadherin-11 regulates cell–cell tension necessary for calcific nodule formation by valvular myofibroblasts. Arterioscler. Thromb. Vasc. Biol. 33, 114–120 (2013).
Garg, V. et al. Mutations in NOTCH1 cause aortic valve disease. Nature 437, 270–274 (2005).
Niessen, K. & Karsan, A. Notch signaling in cardiac development. Circ. Res. 102, 1169–1181 (2008).
Ladich, E., Nakano, M., Carter-Monroe, N. & Virmani, R. Pathology of calcific aortic stenosis. Future Cardiol. 7, 629–642 (2011).
Sun, L., Chandra, S. & Sucosky, P. Ex vivo evidence for the contribution of hemodynamic shear stress abnormalities to the early pathogenesis of calcific bicuspid aortic valve disease. PLoS ONE 7, e48843 (2012).
de la Pompa, J. L. & Epstein, J. A. Coordinating tissue interactions: Notch signaling in cardiac development and disease. Dev. Cell 22, 244–254 (2012).
Andersson, E. R., Sandberg, R. & Lendahl, U. Notch signaling: simplicity in design, versatility in function. Development 138, 3593–3612 (2011).
Tang, Y., Urs, S. & Liaw, L. Hairy-related transcription factors inhibit Notch-induced smooth muscle α-actin expression by interfering with Notch intracellular domain/CBF-1 complex interaction with the CBF-1-binding site. Circ. Res. 102, 661–668 (2008).
Sjolund, J. et al. The notch and TGF-β signaling pathways contribute to the aggressiveness of clear cell renal cell carcinoma. PLoS ONE 6, e23057 (2011).
Tang, Y. et al. Notch and transforming growth factor-β (TGFβ) signaling pathways cooperatively regulate vascular smooth muscle cell differentiation. J. Biol. Chem. 285, 17556–17563 (2010).
Masuda, S. et al. Notch1 oncoprotein antagonizes TGF-β /Smad-mediated cell growth suppression via sequestration of coactivator p300. Cancer Sci. 96, 274–282 (2005).
Sun, Y. et al. Notch4 intracellular domain binding to Smad3 and inhibition of the TGF-β signaling. Oncogene 24, 5365–5374 (2005).
Fu, Y. et al. Differential regulation of transforming growth factor β signaling pathways by Notch in human endothelial cells. J. Biol. Chem. 284, 19452–19462 (2009).
Kennard, S., Liu, H. & Lilly, B. Transforming growth factor-β (TGF-β1) down-regulates Notch3 in fibroblasts to promote smooth muscle gene expression. J. Biol. Chem. 283, 1324–1333 (2008).
Nigam, V. & Srivastava, D. Notch1 represses osteogenic pathways in aortic valve cells. J. Mol. Cell. Cardiol. 47, 828–834 (2009).
Nus, M. et al. Diet-induced aortic valve disease in mice haploinsufficient for the Notch pathway effector RBPJK/CSL. Arterioscler. Thromb. Vasc. Biol. 31, 1580–1588 (2011).
Yang, X. et al. Pro-osteogenic phenotype of human aortic valve interstitial cells is associated with higher levels of Toll-like receptors 2 and 4 and enhanced expression of bone morphogenetic protein 2. J. Am. Coll. Cardiol. 53, 491–500 (2009).
Meng, X. et al. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am. J. Physiol. Cell Physiol. 294, C29–C35 (2008).
Zeng, Q. et al. Notch1 promotes the pro-osteogenic response of human aortic valve interstitial cells via modulation of ERK1/2 and nuclear factor-κB activation. Arterioscler. Thromb. Vasc. Biol. 33, 1580–1590 (2013).
Côté, N. et al. ATP acts as a survival signal and prevents the mineralization of aortic valve. J. Mol. Cell. Cardiol. 52, 1191–1202 (2012).
Mahmut, A. et al. Elevated expression of Lp-PLA2 in calcific aortic valve disease: implication for valve mineralization. J. Am. Coll. Cardiol. http://dx.doi.org/10.1016/j.jacc.2013.05.105.
Osman, L., Chester, A. H., Amrani, M., Yacoub, M. H. & Smolenski, R. T. A novel role of extracellular nucleotides in valve calcification: a potential target for atorvastatin. Circulation 114 (Suppl.), I566–I572 (2006).
Aikawa, E. et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation 119, 1785–1794 (2009).
Hinton, R. B. et al. Elastin haploinsufficiency results in progressive aortic valve malformation and latent valve disease in a mouse model. Circ. Res. 107, 549–557 (2010).
Helske, S. et al. Increased expression of elastolytic cathepsins S, K, and V and their inhibitor cystatin C in stenotic aortic valves. Arterioscler. Thromb. Vasc. Biol. 26, 1791–1798 (2006).
Balachandran, K., Sucosky, P., Jo, H. & Yoganathan, A. P. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. Am. J. Physiol. Heart Circ. Physiol. 296, H756–H764 (2009).
Aikawa, E. & Otto, C. M. Look more closely at the valve: imaging calcific aortic valve disease. Circulation 125, 9–11 (2012).
Aikawa, E. et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation 113, 1344–1352 (2006).
Author information
Authors and Affiliations
Contributions
J. D. Hutcheson researched data for the article, and all the authors contributed substantially to discussion of its content. J. D. Hutcheson and W. D. Merryman wrote the manuscript, and all the authors revised/edited it before submission.
Corresponding author
Ethics declarations
Competing interests
J. D. Hutcheson and W. D. Merryman declare that they have received grant support from the AHA. E. Aikawa and W. D. Merryman declare that they have received grant support from the NIH National Heart, Lung, and Blood Institute.
Rights and permissions
About this article
Cite this article
Hutcheson, J., Aikawa, E. & Merryman, W. Potential drug targets for calcific aortic valve disease. Nat Rev Cardiol 11, 218–231 (2014). https://doi.org/10.1038/nrcardio.2014.1
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2014.1
This article is cited by
-
Challenges of aortic valve tissue culture – maintenance of viability and extracellular matrix in the pulsatile dynamic microphysiological system
Journal of Biological Engineering (2023)
-
Screening of immune-related secretory proteins linking chronic kidney disease with calcific aortic valve disease based on comprehensive bioinformatics analysis and machine learning
Journal of Translational Medicine (2023)
-
Nuclear magnetic resonance spectroscopy to quantify major extracellular matrix components in fibro-calcific aortic valve disease
Scientific Reports (2023)
-
Exosomal miR-129 and miR-342 derived from intermittent hypoxia-stimulated vascular smooth muscle cells inhibit the eIF2α/ATF4 axis from preventing calcified aortic valvular disease
Journal of Cell Communication and Signaling (2023)
-
Subclinical atherosclerosis in primary Sjögren’s syndrome: comparable risk with diabetes mellitus
Clinical Rheumatology (2023)