Key Points
-
Radiotherapy is a common treatment option for cancer patients. However, many aspects of the tumour microenvironment (TME) can render a tumour resistant to radiotherapy de novo or can lead it to recur with a worse prognosis following therapy.
-
Normal tissue toxicity limits the dose of radiotherapy that can be safely delivered.
-
Combination strategies are required in order to achieve better tumour control.
-
Radiotherapy-mediated immunogenic cell death (ICD) can elicit an immune response, but antitumour immunity may be limited owing to the presence of radioresistant suppressor cell types in the TME. Combining radiotherapy and immunomodulatory treatments may overcome adaptive immune suppression and holds great promise both locally in the primary tumour and abscopally.
-
Hypoxia has a crucial role in radioresistance owing to reduced oxygen-mediated fixation of DNA damage and hypoxia induced factor 1α (HIF1α)-mediated cell survival. Attempts to increase oxygen delivery, normalize tumour vessels, inhibit HIF1α and prevent the recruitment of bone marrow-derived cells (BMDCs) required for vasculogenesis are all being tested to reduce tumour hypoxia, improve radiotherapy responses and prevent tumour recurrence after therapy.
-
Tumour irradiation induces a wound healing response that is characterized by inflammation, cancer-associated fibroblast (CAF) modulation and extracellular matrix (ECM) remodelling, which may facilitate tumour recurrence. Targeting the initial inflammatory response may counteract attempts to boost the immune-mediated antitumour response following radiotherapy. Therefore, reducing ECM remodelling by inhibiting growth factors, receptor kinases or matrix enzymes may be more effective in preventing the post-irradiation stiffening of the TME that could facilitate tumour spread.
-
Careful scheduling of tumour reoxygenation strategies with radiotherapy will be required to maximize tumour control. Subsequent inclusion of immunomodulatory and anti-fibrotic treatments should be considered to maximize therapeutic benefits and to prevent post-irradiation tumour recurrence and metastasis.
Abstract
Radiotherapy plays a central part in curing cancer. For decades, most research on improving treatment outcomes has focused on modulating radiation-induced biological effects on cancer cells. Recently, we have better understood that components within the tumour microenvironment have pivotal roles in determining treatment outcomes. In this Review, we describe vascular, stromal and immunological changes that are induced in the tumour microenvironment by irradiation and discuss how these changes may promote radioresistance and tumour recurrence. We also highlight how this knowledge is guiding the development of new treatment paradigms in which biologically targeted agents will be combined with radiotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
24 July 2015
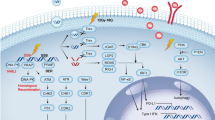
In the version of this article that was originally published, there was an error in Figure 1 on page 410 and in Figure 4 on page 417. In both figures, programmed cell death protein 1 (PD1) was shown to be expressed on the cancer cell and PD1 ligand 1 (PDL1) was shown to be expressed on T cells, whereas PD1 should be expressed on T cells and PDL1 on cancer cells. These errors have now been corrected in the online versions of the article.
References
Harrington, K. J. et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br. J. Cancer 105, 628–639 (2011).
Camphausen, K. & Tofilon, P. J. Combining radiation and molecular targeting in cancer therapy. Cancer Biol. Ther. 3, 247–250 (2004).
Barcellos-Hoff, M. H., Park, C. & Wright, E. G. Radiation and the microenvironment — tumorigenesis and therapy. Nat. Rev. Cancer 5, 867–875 (2005).
Demaria, S., Bhardwaj, N., McBride, W. H. & Formenti, S. C. Combining radiotherapy and immunotherapy: a revived partnership. Int. J. Radiat. Oncol. Biol. Phys. 63, 655–666 (2005).
Durand, R. E. The influence of microenvironmental factors during cancer therapy. In Vivo 8, 691–702 (1994).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Barcellos-Hoff, M. H. & Ravani, S. A. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 60, 1254–1260 (2000). This paper demonstrates that radiotherapy-mediated changes in ECM composition and growth factor activities in the TME can contribute to neoplastic progression.
Good, J. S. & Harrington, K. J. The hallmarks of cancer and the radiation oncologist: updating the 5Rs of radiobiology. Clin. Oncol. (R. Coll. Radiol) 25, 569–577 (2013).
Heckmann, M., Douwes, K., Peter, R. & Degitz, K. Vascular activation of adhesion molecule mRNA and cell surface expression by ionizing radiation. Exp. Cell Res. 238, 148–154 (1998).
Langley, R. E., Bump, E. A., Quartuccio, S. G., Medeiros, D. & Braunhut, S. J. Radiation-induced apoptosis in microvascular endothelial cells. Br. J. Cancer 75, 666–672 (1997).
Paris, F. et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293, 293–297 (2001).
Wang, J., Boerma, M., Fu, Q. & Hauer-Jensen, M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J. Gastroenterol. 13, 3047–3055 (2007).
Baker, D. G. & Krochak, R. J. The response of the microvascular system to radiation: a review. Cancer Invest. 7, 287–294 (1989).
Gujral, D. M., Chahal, N., Senior, R., Harrington, K. J. & Nutting, C. M. Radiation-induced carotid artery atherosclerosis. Radiother. Oncol. 110, 31–38 (2014).
Hoving, S. et al. Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE(−/−) mice. Int. J. Radiat. Oncol. Biol. Phys. 71, 848–857 (2008).
Russell, N. S. et al. Novel insights into pathological changes in muscular arteries of radiotherapy patients. Radiother. Oncol. 92, 477–483 (2009).
Stewart, F. A. et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE−/− mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am. J. Pathol. 168, 649–658 (2006).
Chin, M. S. et al. Skin perfusion and oxygenation changes in radiation fibrosis. Plast. Reconstr. Surg. 131, 707–716 (2013).
Park, H. J., Griffin, R. J., Hui, S., Levitt, S. H. & Song, C. W. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat. Res. 177, 311–327 (2012).
Jain, R. K. Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 (2003).
Denekamp, J. Vascular endothelium as the vulnerable element in tumours. Acta Radiol. Oncol. 23, 217–225 (1984).
Garcia-Barros, M. et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300, 1155–1159 (2003). This paper reports the requirement of ASMase for endothelial cell sensitivity to radiotherapy and also demonstrates that microvascular damage is important for tumour response to radiotherapy.
Ahn, G. O. & Brown, J. M. Influence of bone marrow-derived hematopoietic cells on the tumor response to radiotherapy: experimental models and clinical perspectives. Cell Cycle 8, 970–976 (2009).
Begg, A. C., Stewart, F. A. & Vens, C. Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer 11, 239–253 (2011).
Kioi, M. et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Invest. 120, 694–705 (2010). This paper demonstrates that irradiation induces tumour vasculogenesis through HIF1- and CXCL12-mediated BMDC recruitment in a GBM xenograft model.
Kozin, S. V. et al. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 70, 5679–5685 (2010).
Lerman, O. Z. et al. Low-dose radiation augments vasculogenesis signaling through HIF-1-dependent and -independent SDF-1 induction. Blood 116, 3669–3676 (2010).
Gressner, O. A. & Gressner, A. M. Connective tissue growth factor: a fibrogenic master switch in fibrotic liver diseases. Liver Int. 28, 1065–1079 (2008).
Verrecchia, F. & Mauviel, A. Transforming growth factor-β and fibrosis. World J. Gastroenterol. 13, 3056–3062 (2007).
Wynn, T. A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 (2008).
Yarnold, J. & Brotons, M. C. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 97, 149–161 (2010).
Kidd, S. et al. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS ONE 7, e30563 (2012).
Kalluri, R. & Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006).
Spaeth, E. L. et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE 4, e4992 (2009).
Li, H., Fan, X. & Houghton, J. Tumor microenvironment: the role of the tumor stroma in cancer. J. Cell Biochem. 101, 805–815 (2007).
Augsten, M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front. Oncol. 4, 62 (2014).
Catteau, X., Simon, P. & Noel, J. C. Myofibroblastic stromal reaction and lymph node status in invasive breast carcinoma: possible role of the TGF-β1/TGF-βR1 pathway. BMC Cancer 14, 499 (2014).
Lohr, M. et al. Transforming growth factor-β1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 61, 550–555 (2001).
Ronnov-Jessen, L. & Petersen, O. W. Induction of α-smooth muscle actin by transforming growth factor-β 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 68, 696–707 (1993).
Kojima, Y. et al. Autocrine TGF-β and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc. Natl Acad. Sci. USA 107, 20009–20014 (2010).
Liu, Y. et al. IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-β-independent Smad signaling. J. Immunol. 187, 2814–2823 (2011).
Hellevik, T. et al. Cancer-associated fibroblasts from human NSCLC survive ablative doses of radiation but their invasive capacity is reduced. Radiat. Oncol. 7, 59 (2012).
Cordes, N., Seidler, J., Durzok, R., Geinitz, H. & Brakebusch, C. β1-integrin-mediated signaling essentially contributes to cell survival after radiation-induced genotoxic injury. Oncogene 25, 1378–1390 (2006).
Hodkinson, P. S. et al. ECM overrides DNA damage-induced cell cycle arrest and apoptosis in small-cell lung cancer cells through β1 integrin-dependent activation of PI3-kinase. Cell Death Differ. 13, 1776–1788 (2006).
Park, C. C., Zhang, H. J., Yao, E. S., Park, C. J. & Bissell, M. J. β1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 68, 4398–4405 (2008).
Mantoni, T. S., Lunardi, S., Al-Assar, O., Masamune, A. & Brunner, T. B. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res. 71, 3453–3458 (2011).
Carracedo, S. et al. The fibroblast integrin α11β1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J. Biol. Chem. 285, 10434–10445 (2010).
Puthawala, K. et al. Inhibition of integrin αvβ6, an activator of latent transforming growth factor-β, prevents radiation-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 177, 82–90 (2008).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360 (2004).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Philip, M., Rowley, D. A. & Schreiber, H. Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol. 14, 433–439 (2004).
Li, Q., Withoff, S. & Verma, I. M. Inflammation-associated cancer: NF-κB is the lynchpin. Trends Immunol. 26, 318–325 (2005).
Lin, W. W. & Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 117, 1175–1183 (2007).
Hiniker, S. M. et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl. Oncol. 5, 404–407 (2012).
Harris, T. J. et al. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate 68, 1319–1329 (2008).
Laoui, D., Van Overmeire, E., De Baetselier, P., Van Ginderachter, J. A. & Raes, G. Functional relationship between tumor-associated macrophages and macrophage colony-stimulating factor as contributors to cancer progression. Front. Immunol. 5, 489 (2014).
Kachikwu, E. L. et al. Radiation enhances regulatory T cell representation. Int. J. Radiat. Oncol. Biol. Phys. 81, 1128–1135 (2011).
Qu, Y. et al. Gamma-ray resistance of regulatory CD4+CD25+Foxp3+ T cells in mice. Radiat. Res. 173, 148–157 (2010).
Schaue, D. & McBride, W. H. Links between innate immunity and normal tissue radiobiology. Radiat. Res. 173, 406–417 (2010).
Ozsoy, H. Z., Sivasubramanian, N., Wieder, E. D., Pedersen, S. & Mann, D. L. Oxidative stress promotes ligand-independent and enhanced ligand-dependent tumor necrosis factor receptor signaling. J. Biol. Chem. 283, 23419–23428 (2008).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Martins, I. et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene 30, 1147–1158 (2011).
Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875 (2012).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 (2007). This paper explores the immunogenic response to HMGB1 release by dying tumour cells through the PRR TLR4, which is expressed on DCs, after chemotherapy or radiotherapy. It demonstrates the important role of PRR DAMP signalling in efficient DC activation and antigen cross-presentation.
Qureshi, O. S. et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332, 600–603 (2011). This study explores the immunoregulatory actions of CTLA4 against stimulation of CD28 by CD80 and CD86 in vitro and in vivo and therefore provides a biological basis for the efficacy of anti-CTLA4 therapy in cancer treatments.
Meng, Y. et al. Ad.Egr-TNF and local ionizing radiation suppress metastases by interferon-β-dependent activation of antigen-specific CD8+ T cells. Mol. Ther. 18, 912–920 (2010).
Matsumura, S. et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J. Immunol. 181, 3099–3107 (2008).
Burnette, B. C. et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 71, 2488–2496 (2011).
Gupta, A. et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J. Immunol. 189, 558–566 (2012). This study examines the importance of DC activation in generating an effective CD8+ T cell response after radiotherapy.
Antoniades, J., Brady, L. W. & Lightfoot, D. A. Lymphangiographic demonstration of the abscopal effect in patients with malignant lymphomas. Int. J. Radiat. Oncol. Biol. Phys. 2, 141–147 (1977).
Ohba, K. et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut 43, 575–577 (1998).
Wersall, P. J. et al. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 45, 493–497 (2006).
Okuma, K., Yamashita, H., Niibe, Y., Hayakawa, K. & Nakagawa, K. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. J. Med. Case Rep. 5, 111 (2011).
Postow, M. A. et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 366, 925–931 (2012). This case report demonstrates the abscopal effect with the combination of high fraction radiotherapy and anti-CTLA4 immunotherapy in metastatic melanoma, leading to regression of all metastases in the patient. They also noted the occurrence of antibody responses to the cancer testis antigen NY-ESO-1, changes in blood immune cells and increased antibody responses to other antigens following radiotherapy.
Stamell, E. F., Wolchok, J. D., Gnjatic, S., Lee, N. Y. & Brownell, I. The abscopal effect associated with a systemic anti-melanoma immune response. Int. J. Radiat. Oncol. Biol. Phys. 85, 293–295 (2013).
Harris, A. L. Hypoxia — a key regulatory factor in tumour growth. Nat. Rev. Cancer 2, 38–47 (2002).
Overgaard, J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck — a systematic review and meta-analysis. Radiother. Oncol. 100, 22–32 (2011). This reference is a comprehensive review of the importance of tumour hypoxia for radiotherapy outcome and the current methods being trialled to modify hypoxia during radiotherapy for patients with HNSCC.
Vaupel, P., Kallinowski, F. & Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 49, 6449–6465 (1989).
Brown, J. M. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br. J. Radiol. 52, 650–656 (1979).
Dewhirst, M. W. et al. Microvascular studies on the origins of perfusion-limited hypoxia. Br. J. Cancer Suppl. 27, S247–251 (1996).
Harada, H. How can we overcome tumor hypoxia in radiation therapy? J. Radiat. Res. 52, 545–556 (2011).
Semenza, G. L. Intratumoral hypoxia, radiation resistance, and HIF-1. Cancer Cell 5, 405–406 (2004).
Brizel, D. M., Sibley, G. S., Prosnitz, L. R., Scher, R. L. & Dewhirst, M. W. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 38, 285–289 (1997).
Yoshimura, M., Itasaka, S., Harada, H. & Hiraoka, M. Microenvironment and radiation therapy. Biomed. Res. Int. 2013, 685308 (2013).
Janssens, G. O. et al. Improved recurrence-free survival with ARCON for anemic patients with laryngeal cancer. Clin. Cancer Res. 20, 1345–1354 (2014).
Hoskin, P., Rojas, A. & Saunders, M. Accelerated radiotherapy, carbogen, and nicotinamide (ARCON) in the treatment of advanced bladder cancer: mature results of a Phase II nonrandomized study. Int. J. Radiat. Oncol. Biol. Phys. 73, 1425–1431 (2009).
Janssens, G. O. et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a Phase III randomized trial. J. Clin. Oncol. 30, 1777–1783 (2012).
Baillet, F., Housset, M., Dessard-Diana, B. & Boisserie, G. Positive clinical experience with misonidazole in brachytherapy and external radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 16, 1073–1075 (1989).
Minsky, B. D. & Leibel, S. A. The treatment of hepatic metastases from colorectal cancer with radiation therapy alone or combined with chemotherapy or misonidazole. Cancer Treat. Rev. 16, 213–219 (1989).
Simpson, J. R. et al. Radiation therapy alone or combined with misonidazole in the treatment of locally advanced non-oat cell lung cancer: report of an RTOG prospective randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 16, 1483–1491 (1989).
Nishimura, Y. et al. Phase I/II trial of sequential chemoradiotherapy using a novel hypoxic cell radiosensitizer, doranidazole (PR-350), in patients with locally advanced non-small-cell lung Cancer (WJTOG-0002). Int. J. Radiat. Oncol. Biol. Phys. 69, 786–792 (2007).
Overgaard, J. et al. A randomized double-blind Phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5–85. Radiother. Oncol. 46, 135–146 (1998).
Thomson, D. et al. NIMRAD — a Phase III trial to investigate the use of nimorazole hypoxia modification with intensity-modulated radiotherapy in head and neck cancer. Clin. Oncol. (R. Coll. Radiol.) 26, 344–347 (2014).
Prasad, P. et al. Multifunctional albumin–MnO2 nanoparticles modulate solid tumor microenvironment by attenuating hypoxia, acidosis, vascular endothelial growth factor and enhance radiation response. ACS Nano 8, 3202–3212 (2014). This paper introduces the most recent attempt to modify tumour hypoxia using albumin–MnO 2 nanoparticles, which increase tumour oxygenation in hypoxic regions. This treatment enhanced the radiotherapy response of breast cancer.
Moeller, B. J., Cao, Y., Li, C. Y. & Dewhirst, M. W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 5, 429–441 (2004). This paper demonstrates that radiotherapy-mediated tumour reoxygenation stabilizes HIF1, leading to enhanced cytokine secretion and endothelial cell radioresistance.
Aebersold, D. M. et al. Expression of hypoxia-inducible factor-1α: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 61, 2911–2916 (2001).
Koukourakis, M. I. et al. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 53, 1192–1202 (2002).
Koukourakis, M. I. et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 α and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J. Clin. Oncol. 24, 727–735 (2006).
Lee, K. et al. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc. Natl Acad. Sci. USA 106, 17910–17915 (2009).
Harada, H. et al. Treatment regimen determines whether an HIF-1 inhibitor enhances or inhibits the effect of radiation therapy. Br. J. Cancer 100, 747–757 (2009).
Riedel, K. et al. Abrogation of TGF-β by antisense oligonucleotides modulates expression of VEGF and increases angiogenic potential in isolated fibroblasts from radiated skin. Int. J. Mol. Med. 22, 473–480 (2008).
Goumans, M. J. et al. Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J. 21, 1743–1753 (2002).
Carmeliet, P. & Jain, R. K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 10, 417–427 (2011).
Ferrara, N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2, 795–803 (2002).
Goel, S. et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 91, 1071–1121 (2011).
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005). This paper reviews the evidence that was emerging at the time that certain anti-angiogenic agents can normalize the abnormal tumour vessels to enable better oxygen and drug delivery, rather than completely destroying the tumour vasculature as was previously thought.
Fokas, E. et al. Dual inhibition of the PI3K/mTOR pathway increases tumor radiosensitivity by normalizing tumor vasculature. Cancer Res. 72, 239–248 (2012).
Dings, R. P. et al. Scheduling of radiation with angiogenesis inhibitors anginex and Avastin improves therapeutic outcome via vessel normalization. Clin. Cancer Res. 13, 3395–3402 (2007). This paper demonstrates the use of anti-angiogenics in vessel normalization and the importance of scheduling radiotherapy to fall within the tumour oxygenation window.
McGee, M. C. et al. Improved intratumoral oxygenation through vascular normalization increases glioma sensitivity to ionizing radiation. Int. J. Radiat. Oncol. Biol. Phys. 76, 1537–1545 (2010).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Lund, E. L., Bastholm, L. & Kristjansen, P. E. Therapeutic synergy of TNP-470 and ionizing radiation: effects on tumor growth, vessel morphology, and angiogenesis in human glioblastoma multiforme xenografts. Clin. Cancer Res. 6, 971–978 (2000).
Teicher, B. A., Emi, Y., Kakeji, Y. & Northey, D. TNP-470/minocycline/cytotoxic therapy: a systems approach to cancer therapy. Eur. J. Cancer 32A, 2461–2466 (1996).
Teicher, B. A. et al. Potentiation of cytotoxic therapies by TNP-470 and minocycline in mice bearing EMT-6 mammary carcinoma. Breast Cancer Res. Treat. 36, 227–236 (1995).
Ling, Y. et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem. Biophys. Res. Commun. 361, 79–84 (2007).
Wu, Y. et al. Endostar combined with radiotherapy increases radiation sensitivity by decreasing the expression of TGF-β1, HIF-1α and bFGF. Exp. Ther. Med. 7, 911–916 (2014).
Zhou, J. et al. Antitumor activity of Endostar combined with radiation against human nasopharyngeal carcinoma in mouse xenograft models. Oncol. Lett. 4, 976–980 (2012).
Brown, J. M. Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy. Br. J. Radiol 87, 20130686 (2014).
Burrell, K., Singh, S., Jalali, S., Hill, R. P. & Zadeh, G. VEGF regulates region-specific localization of perivascular bone marrow-derived cells in glioblastoma. Cancer Res. 74, 3727–3739 (2014).
Abdollahi, A. et al. Inhibition of αvβ3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin. Cancer Res. 11, 6270–6279 (2005).
Nabors, L. B. et al. A safety run-in and randomized Phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma (NABTT 0306). Cancer 118, 5601–5607 (2012).
Stupp, R. et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, Phase 3 trial. Lancet Oncol. 15, 1100–1108 (2014).
Gutheil, J. C. et al. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin αvβ3. Clin. Cancer Res. 6, 3056–3061 (2000).
Kuwada, S. K. Drug evaluation: volociximab, an angiogenesis-inhibiting chimeric monoclonal antibody. Curr. Opin. Mol. Ther. 9, 92–98 (2007).
Barkan, D. & Chambers, A. F. β1-integrin: a potential therapeutic target in the battle against cancer recurrence. Clin. Cancer Res. 17, 7219–7223 (2011).
Multhoff, G. & Radons, J. Radiation, inflammation, and immune responses in cancer. Front. Oncol. 2, 58 (2012).
Barcellos-Hoff, M. H., Derynck, R., Tsang, M. L. & Weatherbee, J. A. Transforming growth factor-β activation in irradiated murine mammary gland. J. Clin. Invest. 93, 892–899 (1994).
Dancea, H. C., Shareef, M. M. & Ahmed, M. M. Role of radiation-induced TGF-β signaling in cancer therapy. Mol. Cell Pharmacol. 1, 44–56 (2009).
Biswas, S. et al. Inhibition of TGF-β with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J. Clin. Invest. 117, 1305–1313 (2007). This paper implicates TGFβ in post-radiotherapy induction of metastasis and provides a rationale for combining TGFβ inhibitors with radiotherapy to prevent cancer progression.
Bouquet, F. et al. TGFβ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin. Cancer Res. 17, 6754–6765 (2011).
Neuzillet, C. et al. Targeting the TGFβ pathway for cancer therapy. Pharmacology and Therapeutics 147, 22–31 (2014).
Medicherla, S. et al. Antitumor activity of TGF-β inhibitor is dependent on the microenvironment. Anticancer Res. 27, 4149–4157 (2007).
Cui, J. J. Targeting receptor tyrosine kinase MET in cancer: small molecule inhibitors and clinical progress. J. Med. Chem. 57, 4427–4453 (2014).
De Bacco, F. et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J. Natl Cancer Inst. 103, 645–661 (2011).
Ohuchida, K. et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 64, 3215–3222 (2004). This paper presents the findings that irradiated fibroblasts can increase the invasiveness of pancreatic cancer cells. This increased invasion can be abrogated through MET inhibition using a specific HGF antagonist.
Cokgor, I. et al. Phase I trial results of iodine-131-labeled antitenascin monoclonal antibody 81C6 treatment of patients with newly diagnosed malignant gliomas. J. Clin. Oncol. 18, 3862–3872 (2000).
Reardon, D. A. et al. Phase II trial of murine 131I-labeled antitenascin monoclonal antibody 81C6 administered into surgically created resection cavities of patients with newly diagnosed malignant gliomas. J. Clin. Oncol. 20, 1389–1397 (2002).
Reardon, D. A. et al. A pilot study: 131I-antitenascin monoclonal antibody 81c6 to deliver a 44-Gy resection cavity boost. Neuro Oncol. 10, 182–189 (2008).
Distler, J. H. & Distler, O. Tyrosine kinase inhibitors for the treatment of fibrotic diseases such as systemic sclerosis: towards molecular targeted therapies. Ann. Rheum. Dis. 69 (Suppl. 1), i48–i51 (2010).
Antoniu, S. A. Nintedanib (BIBF 1120) for IPF: a tomorrow therapy? Multidiscip. Respir. Med. 7, 41 (2012).
Chaudhary, N. I. et al. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur. Respir. J. 29, 976–985 (2007).
Omenetti, A. et al. Hedgehog signaling regulates epithelial–mesenchymal transition during biliary fibrosis in rodents and humans. J. Clin. Invest. 118, 3331–3342 (2008).
Bailey, J. M. et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin. Cancer Res. 14, 5995–6004 (2008).
Lee, M. J. et al. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proc. Natl Acad. Sci. USA 109, 7859–7864 (2012).
Rodon, J. et al. A Phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin. Cancer Res. 20, 1900–1909 (2014).
Sekulic, A. et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 366, 2171–2179 (2012).
Bayers, S., Kapp, D. L., Beer, K. R. & Slavin, B. Treatment of margin positive basal cell carcinoma with vismodegib: case report and consideration of treatment options and their implications. J. Drugs Dermatol. 12, s147–150 (2013).
Leschey, K. H., Hines, J., Singer, J. H., Hackett, S. F. & Campochiaro, P. A. Inhibition of growth factor effects in retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 32, 1770–1778 (1991).
McGeary, R. P., Bennett, A. J., Tran, Q. B., Cosgrove, K. L. & Ross, B. P. Suramin: clinical uses and structure-activity relationships. Mini Rev. Med. Chem. 8, 1384–1394 (2008).
Laterra, J. J. et al. Suramin and radiotherapy in newly diagnosed glioblastoma: Phase 2 NABTT CNS Consortium study. Neuro Oncol. 6, 15–20 (2004).
Tayel, A. et al. Suramin inhibits hepatic tissue damage in hepatocellular carcinoma through deactivation of heparanase enzyme. Eur. J. Pharmacol. 728, 151–160 (2014).
Vlodavsky, I. et al. Significance of heparanase in cancer and inflammation. Cancer Microenviron 5, 115–132 (2012).
Meirovitz, A. et al. Role of heparanase in radiation-enhanced invasiveness of pancreatic carcinoma. Cancer Res. 71, 2772–2780 (2011). This article reports that radiotherapy-induced heparanase upregulation increases the invasion of pancreatic cancer cells. Inhibition of heparanase using SST0001 in combination with radiotherapy successfully attenuated the spread of orthotopic pancreatic tumours in vivo.
Dredge, K. et al. PG545, a dual heparanase and angiogenesis inhibitor, induces potent anti-tumour and anti-metastatic efficacy in preclinical models. Br. J. Cancer 104, 635–642 (2011).
Liu, C. J. et al. Adjuvant heparanase inhibitor PI-88 therapy for hepatocellular carcinoma recurrence. World J. Gastroenterol. 20, 11384–11393 (2014).
Hammond, E., Handley, P., Dredge, K. & Bytheway, I. Mechanisms of heparanase inhibition by the heparan sulfate mimetic PG545 and three structural analogues. FEBS Open Bio 3, 346–351 (2013).
Junttila, M. R. & de Sauvage, F. J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501, 346–354 (2013).
Badiga, A. V. et al. MMP-2 siRNA inhibits radiation-enhanced invasiveness in glioma cells. PLoS ONE 6, e20614 (2011).
Kaliski, A. et al. Angiogenesis and tumor growth inhibition by a matrix metalloproteinase inhibitor targeting radiation-induced invasion. Mol. Cancer Ther. 4, 1717–1728 (2005).
Qian, L. W. et al. Radiation-induced increase in invasive potential of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor, CGS27023. Clin. Cancer Res. 8, 1223–1227 (2002).
Takahashi, M. et al. In vivo glioma growth requires host-derived matrix metalloproteinase 2 for maintenance of angioarchitecture. Pharmacol. Res. 46, 155–163 (2002).
Özdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014). This paper demonstrates a novel role for CAFs in the TME of pancreatic cancer. In contrast to previous beliefs, the depletion of CAFs in a pancreatic cancer model led to disease progression. Moreover, patients with pancreatic ductal adenocarcinoma with fewer myofibroblasts were shown to have a worse prognosis.
Huang, Q. et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 17, 860–866 (2011). This study identifies caspase 3, which is involved in apoptosis after exposure to ionizing radiation, as an important component in repopulation signalling and tumour resistance.
Mantovani, A., Romero, P., Palucka, A. K. & Marincola, F. M. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 371, 771–783 (2008).
Finkelstein, S. E. et al. Combination of external beam radiotherapy (EBRT) with intratumoral injection of dendritic cells as neo-adjuvant treatment of high-risk soft tissue sarcoma patients. Int. J. Radiat. Oncol. Biol. Phys. 82, 924–932 (2012).
Roses, R. E., Datta, J. & Czerniecki, B. J. Radiation as immunomodulator: implications for dendritic cell-based immunotherapy. Radiat. Res. 182, 211–218 (2014).
Dewan, M. Z. et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin. Cancer Res. 18, 6668–6678 (2012). This study explores different fractionation of radiotherapy in combination with CTLA4-specific antibodies in a murine model, demonstrating an optimal fractionation at around 24 Gy in three doses.
Czerniecki, B. J. et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 67, 1842–1852 (2007).
Formenti, S. et al. Pilot trial of radiation therapy and GM-CSF in metastatic cancer: abscopal responses. Int. J. Radi. Oncol. Biol. Phys. 84, S178–S178 (2012).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Robert, C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011).
Dewan, M. Z. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009).
Gough, M. J. et al. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J. Immunother. 33, 798–809 (2010).
de la Cruz-Merino, L. et al. Radiation for awakening the dormant immune system, a promising challenge to be explored. Front. Immunol. 5, 102 (2014).
Melero, I., Hervas-Stubbs, S., Glennie, M., Pardoll, D. M. & Chen, L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat. Rev. Cancer 7, 95–106 (2007).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Baitsch, L. et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360 (2011).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010). This study looks at the reversal of T cell exhaustion by combined targeting of PD1 and TIM3 receptors as a means of increasing tumour immunity. It is likely that similar combinations in conjunction with radiotherapy could prove to be effective as future cancer treatments.
Matsuzaki, J. et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl Acad. Sci. USA 107, 7875–7880 (2010).
Deng, L. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 124, 687–695 (2014). This preclinical study demonstrates the effectiveness of targeting T cell exhaustion pathways in combination with radiotherapy. There is also a potential synergistic effect with anti-CTLA4 therapy and other immunomodulatory therapies.
Verbrugge, I. et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 72, 3163–3174 (2012).
Marabelle, A., Filatenkov, A., Sagiv-Barfi, I. & Kohrt, H. Radiotherapy and toll-like receptor agonists. Semin. Radiat. Oncol. 25, 34–39 (2015).
Russell, S. J., Peng, K. W. & Bell, J. C. Oncolytic virotherapy. Nat. Biotech. 30, 658–670 (2012).
Prestwich, R. J. et al. Oncolytic viruses: a novel form of immunotherapy. Expert Rev. Anticancer Ther. 8, 1581–1588 (2008).
Dai, M. H. et al. Oncolytic vaccinia virus in combination with radiation shows synergistic antitumor efficacy in pancreatic cancer. Cancer Lett. 344, 282–290 (2014).
Kyula, J. N. et al. Synergistic cytotoxicity of radiation and oncolytic Lister strain vaccinia in (V600D/E)BRAF mutant melanoma depends on JNK and TNF-α signaling. Oncogene 33, 1700–1712 (2014).
Harrington, K. J. et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin. Cancer Res. 16, 4005–4015 (2010). This study looks at using oncolytic virus with immunomodulation and radiotherapy and chemotherapy to treat resistant HNSCC, demonstrating the combinatorial possibilities available with oncoviral therapy in treating resistant cancers.
Miyamoto, S. et al. Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res. 72, 2609–2621 (2012).
Hu, J. C. et al. A Phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 12, 6737–6747 (2006).
Brown, J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 4, 437–447 (2004).
Zeng, J. et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 86, 343–349 (2013).
Demaria, S. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005).
Chi, K. H. et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J. Immunother. 28, 129–135 (2005).
Gulley, J. L. et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin. Cancer Res. 11, 3353–3362 (2005).
Kim, Y. H. et al. Phase I trial of a Toll-like receptor 9 agonist, PF-3512676 (CPG 7909), in patients with treatment-refractory, cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 63, 975–983 (2010).
Seung, S. K. et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2 — tumor and immunological responses. Sci. Transl Med. 4, 137ra74 (2012).
Acknowledgements
The authors acknowledge support from Cancer Research UK programme grants C46/A10588 and C7224/A13407, the Wellcome Trust, the NIHR Royal Marsden/Institute of Cancer Research Biomedical Research Centre, Oracle Cancer Trust, Rosetrees Trust and Anthony Long Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
DATABASES
Glossary
- COMMA-D cells
-
An epithelial cell line derived from the mammary tissue of a mid-pregnant BALB/c mouse, which exhibits many characteristics distinctive of normal mammary epithelial cells.
- Atherosclerosis
-
Thickening of the artery wall as a result of white blood cell invasion and accumulation.
- Medial necrosis
-
Necrosis of the middle portion of vessel walls (anatomically called the tunica media).
- Vessel co-option
-
A mechanism by which tumours obtain a blood supply by hijacking the existing vasculature.
- Microvascular injury
-
Injury to the fine network of blood vessels and capillaries that results in changed patterns of blood flow.
- Desmoplastic reaction
-
A stromal reaction that can be induced by tissue injury, wound repair or cancer growth. Increased extracellular matrix and growth factor production and secretion result in the formation of scar-like fibrotic tissue.
- Adaptive immunity
-
A carefully regulated, adaptable, specific immune response comprising humoral- and cell-mediated components. It is capable of both systemic actions and immunological memory to specific stimuli. It is triggered by known antigens or by appropriate antigen presentation from the innate immune system.
- Tumour associated macrophages
-
(TAMs). Macrophages within the tumour microenvironment; they are generally immunosuppressive and resemble the alternatively activated M2 macrophage.
- Regulatory T cells
-
(TReg cells). A T cell subset that exerts immunosuppressive and tolerizing effects. TReg cells have an important role in cancer immune editing, in the maintenance of a permissive cancer microenvironment and in preventing effective adaptive immune recognition of tumour cells.
- Innate immune system
-
The initial immune response that occurs in a generic manner to inflammatory stimuli, comprising complement activation and immune cell recruitment and activation. It coordinates the activation of the adaptive immune system by antigen presentation.
- Direct and indirect radiation effects
-
Radiation damage can be divided into direct effects, where the damage is a result of the ionizing radiation itself, and indirect effects, which refer to the resultant changes in cellular pathways as a result of radiation: for example, as a result of reactive oxygen species.
- Damage-associated molecular patterns
-
(DAMPs). Stimuli released by stressed, dying or injured cells that may trigger an inflammatory response by the activation of a number of pattern recognition receptors.
- Pathogen-associated molecular patterns
-
(PAMPs). Signalling from pathogens by particular stimuli that can be recognized by immune cells, leading to an inflammatory response.
- Immunogenic cell death
-
Cell death that triggers an immune reaction by DAMP–PRR signalling. This may occur as a result of different types of tissue damage; for example, damage caused by radiotherapy or chemotherapy.
- Immune tolerization
-
The recognition of 'self' and 'non-self' during antigen presentation is important and carefully regulated to prevent autoimmunity, it is the process of recognizing antigens as 'self'.
- Abscopal effects
-
Irradiation of tumour cells or the adjacent extracellular matrix can induce biologically relevant changes in distant cells, which may or may not have been irradiated themselves. These are distinguished from bystander (which refers to changes affecting nearby unirradiated cells) and cohort effects (which refer to changes affecting off-target irradiated cells).
- Eccrine
-
A type of tumour from secretory sweat glands.
Rights and permissions
About this article
Cite this article
Barker, H., Paget, J., Khan, A. et al. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 15, 409–425 (2015). https://doi.org/10.1038/nrc3958
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3958
This article is cited by
-
FBW7/GSK3β mediated degradation of IGF2BP2 inhibits IGF2BP2-SLC7A5 positive feedback loop and radioresistance in lung cancer
Journal of Experimental & Clinical Cancer Research (2024)
-
Synergistic cerium oxide nanozymes: targeting DNA damage and alleviating tumor hypoxia for improved NSCLC radiotherapy efficiency
Journal of Nanobiotechnology (2024)
-
Microenvironmental reorganization in brain tumors following radiotherapy and recurrence revealed by hyperplexed immunofluorescence imaging
Nature Communications (2024)
-
Impact of LKB1 status on radiation outcome in patients with stage III non-small-cell lung cancer
Scientific Reports (2024)
-
Role of the mechanical microenvironment on CD-44 expression of breast adenocarcinoma in response to radiotherapy
Scientific Reports (2024)