Key Points
-
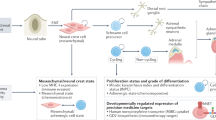
Neuroblastoma is a heterogeneous disease. Over 60% of neuroblastomas are metastatic, and most are diagnosed after 18 months of age, with a substantial number carrying MYCN amplification or α-thalassaemia/mental retardation syndrome X-linked (ATRX) mutation, and/or anaplastic lymphoma receptor tyrosine kinase (ALK) mutation. The rest have fairly few somatic mutations and are highly curable with either surgery alone or surgery and low-dose chemotherapy. Neural crest cells and neuroblastoma share common pathways and genes, including paired-like homeobox 2b (PHOX2B), MYCN and ALK.
-
A predictive profile of genetic predisposition to neuroblastoma is emerging via genome-wide association and whole-genome sequencing analyses. However, in contrast to adult cancers, there is a general paucity of recurrent somatic mutations in neuroblastoma.
-
The biology of catecholamine transport has been successfully exploited to provide the tumour-specific neurotransmitter analogue meta-iodobenzylguanidine (MIBG) for diagnosis and anti-neuroblastoma therapy. This advance shows how understanding unique tumour physiology can lead to new therapeutics that are not directly related to specific genetic lesions.
-
Chromosomal aberration is common in neuroblastoma; numerical whole-chromosomal gains are typically found in low-risk tumours, whereas segmental chromosomal gains or losses and somatic mutations are associated with high-risk disease.
-
Research on epigenetic regulation and microRNA control may uncover new prognostic markers and therapeutic targets for neuroblastoma.
-
Neuroblastoma can evade T cells and natural killer cells while exploiting inflammatory macrophages to enhance its survival. Monoclonal antibodies, cytokines and multifunctional antibodies could potentially reactivate antitumour activity in these cells.
-
Anti-GD2 antibodies, when combined with granulocyte–macrophage colony-stimulating factor with or without interleukin-2, are one of the most successful and important strategies for the curative approach to neuroblastoma. Both myeloid effectors and natural killer cells and their cell-surface activating or inhibitory receptors have crucial roles in the clinical response.
Abstract
Neuroblastoma is a solid tumour that arises from the developing sympathetic nervous system. Over the past decade, our understanding of this disease has advanced tremendously. The future challenge is to apply the knowledge gained to developing risk-based therapies and, ultimately, improving outcome. In this Review we discuss the key discoveries in the developmental biology, molecular genetics and immunology of neuroblastoma, as well as new translational tools for bringing these promising scientific advances into the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Maris, J. M. Recent advances in neuroblastoma. N. Engl. J. Med. 362, 2202–2211 (2010).
Brodeur, G. M. Neuroblastoma: biological insights into a clinical enigma. Nature Rev. Cancer 3, 203–216 (2003).
Cohn, S. L. et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J. Clin. Oncol. 27, 289–297 (2009). The current guidelines for classifying risk groups among patients with neuroblastoma.
Molenaar, J. J. et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 483, 589–593 (2012).
Cheung, N. K. et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. J. Am. Med. Assoc. 307, 1062–1071 (2012). References 4 and 5 were the first reports of whole-genome sequencing of neuroblastoma and identify ATRX as a recurrent genetic lesion in neuroblastoma.
Pugh, T. J. et al. The genetic landscape of high-risk neuroblastoma. Nature Genet. 45, 279–284 (2013).
Sausen, M. et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nature Genet. 45, 12–17 (2012).
Segal, N. H. et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 68, 889–892 (2008).
Grupp, S. A., Asgharzadeh, S. & Yanik, G. A. Neuroblastoma: issues in transplantation. Biol. Blood Marrow Transplant 18, S92–S100 (2012).
Anderson, D. J. & Axel, R. A bipotential neuroendocrine precursor whose choice of cell fate is determined by NGF and glucocorticoids. Cell 47, 1079–1090 (1986). This study established the early lineage relationships in neural crest development.
Anderson, D. J., Carnahan, J. F., Michelsohn, A. & Patterson, P. H. Antibody markers identify a common progenitor to sympathetic neurons and chromaffin cells in vivo and reveal the timing of commitment to neuronal differentiation in the sympathoadrenal lineage. J. Neurosci. 11, 3507–3519 (1991). These authors demonstrated that the tyrosine hydroxylase gene is expressed in precursors of the adrenal and paraspinal ganglia, and they used this information to produce the first mouse model of neuroblastoma.
Le Dourin, N. M. & Kalcheim, G. The Neural Crest (Cambridge Univ. Press, 1999).
Pages, P. M. et al. Bilateral adrenal neuroblastoma. Pediatr. Blood Cancer 52, 196–202 (2009).
Knudson, A. G. Jr & Strong, L. C. Mutation and cancer: neuroblastoma and pheochromocytoma. Am. J. Hum. Genet. 24, 514–532 (1972).
Maris, J. M. et al. Evidence for a hereditary neuroblastoma predisposition locus at chromosome 16p12-13. Cancer Res. 62, 6651–6658 (2002).
Mosse, Y. P. et al. Germline PHOX2B mutation in hereditary neuroblastoma. Am. J. Hum. Genet. 75, 727–730 (2004).
Trochet, D. et al. Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma. Am. J. Hum. Genet. 74, 761–764 (2004). The first description of the association between germline PHOX2B mutation and hereditary neuroblastoma.
Trochet, D. et al. Molecular consequences of PHOX2B missense, frameshift and alanine expansion mutations leading to autonomic dysfunction. Hum. Mol. Genet. 14, 3697–3708 (2005).
Raabe, E. H. et al. Prevalence and functional consequence of PHOX2B mutations in neuroblastoma. Oncogene 27, 469–476 (2008).
Stovroff, M., Dykes, F. & Teague, W. G. The complete spectrum of neurocristopathy in an infant with congenital hypoventilation, Hirschsprung's disease, and neuroblastoma. J. Pediatr. Surg. 30, 1218–1221 (1995).
Wilzen, A. et al. The Phox2 pathway is differentially expressed in neuroblastoma tumors, but no mutations were found in the candidate tumor suppressor gene PHOX2A. Int. J. Oncol. 34, 697–705 (2009).
Nagashimada, M. et al. Autonomic neurocristopathy-associated mutations in PHOX2B dysregulate Sox10 expression. J. Clin. Invest. 122, 3145–3158 (2012).
Mosse, Y. P. et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455, 930–935 (2008).
Janoueix-Lerosey, I. et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 455, 967–970 (2008).
Chen, Y. et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature 455, 971–974 (2008).
George, R. E. et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 455, 975–978 (2008). References 23–26 reported the identification of ALK mutations in familial and sporadic neuroblastoma.
Wellstein, A. ALK receptor activation, ligands and therapeutic targeting in glioblastoma and in other cancers. Front. Oncol. 2, 192 (2012).
Iwahara, T. et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 14, 439–449 (1997).
Degoutin, J., Brunet-de Carvalho, N., Cifuentes-Diaz, C. & Vigny, M. ALK (anaplastic lymphoma kinase) expression in DRG neurons and its involvement in neuron-Schwann cells interaction. Eur. J. Neurosci. 29, 275–286 (2009).
Souttou, B., Carvalho, N. B., Raulais, D. & Vigny, M. Activation of anaplastic lymphoma kinase receptor tyrosine kinase induces neuronal differentiation through the mitogen-activated protein kinase pathway. J. Biol. Chem. 276, 9526–9531 (2001).
Motegi, A., Fujimoto, J., Kotani, M., Sakuraba, H. & Yamamoto, T. ALK receptor tyrosine kinase promotes cell growth and neurite outgrowth. J. Cell Sci. 117, 3319–3329 (2004).
Schonherr, C., Yang, H. L., Vigny, M., Palmer, R. H. & Hallberg, B. Anaplastic lymphoma kinase activates the small GTPase Rap1 via the Rap1-specific GEF C3G in both neuroblastoma and PC12 cells. Oncogene 29, 2817–2830 (2010).
Bachetti, T. et al. PHOX2B-mediated regulation of ALK expression: in vitro identification of a functional relationship between two genes involved in neuroblastoma. PLoS ONE 5, e13108 (2010).
Reiff, T. et al. Midkine and Alk signaling in sympathetic neuron proliferation and neuroblastoma predisposition. Development 138, 4699–4708 (2011).
Berry, T. et al. The ALKF1174L mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell 22, 117–130 (2012).
Heukamp, L. C. et al. Targeted expression of mutated ALK induces neuroblastoma in transgenic mice. Sci. Transl. Med. 4, 141ra91 (2012).
Schulte, J. H. et al. MYCN and ALKF1174L are sufficient to drive neuroblastoma development from neural crest progenitor cells. Oncogene 32, 1059–1065 (2012). References 35–37 reported that ALK-activating mutations contributed to neuroblastoma tumorigenesis in mice.
Schulte, J. H. et al. High ALK receptor tyrosine kinase expression supersedes ALK mutation as a determining factor of an unfavorable phenotype in primary neuroblastoma. Clin. Cancer Res. 17, 5082–5092 (2011).
Carpenter, E. L. & Mosse, Y. P. Targeting ALK in neuroblastoma—preclinical and clinical advancements. Nature Rev. Clin. Oncol. 9, 391–399 (2012).
Grimmer, M. R. & Weiss, W. A. Childhood tumors of the nervous system as disorders of normal development. Curr. Opin. Pediatr. 18, 634–638 (2006).
Zhu, S. et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell 21, 362–373 (2012).
Weiss, W. A., Aldape, K., Mohapatra, G., Feuerstein, B. G. & Bishop, J. M. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 16, 2985–2995 (1997). The first description of MYCN causing neuroblastoma in transgenic mice.
Brodeur, G. M., Seeger, R. C., Schwab, M., Varmus, H. E. & Bishop, J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 224, 1121–1124 (1984). A report of MYCN amplification in neuroblastoma and its correlation with disease outcome.
Faisal, A. et al. The aurora kinase inhibitor CCT137690 downregulates MYCN and sensitizes MYCN-amplified neuroblastoma in vivo. Mol. Cancer Ther. 10, 2115–2123 (2011).
Puissant, A. et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 3, 308–323 (2013).
Blackburn, E. H. Telomeres and telomerase: the means to the end (Nobel lecture). Angew. Chem. Int. Ed Engl. 49, 7405–7421 (2010).
Poremba, C. et al. Telomerase activity and telomerase subunits gene expression patterns in neuroblastoma: a molecular and immunohistochemical study establishing prognostic tools for fresh-frozen and paraffin-embedded tissues. J. Clin. Oncol. 18, 2582–2592 (2000).
Hiyama, E. et al. Correlating telomerase activity levels with human neuroblastoma outcomes. Nature Med. 1, 249–255 (1995).
Coco, S. et al. Age-dependent accumulation of genomic aberrations and deregulation of cell cycle and telomerase genes in metastatic neuroblastoma. Int. J. Cancer 131, 1591–1600 (2012).
Onitake, Y. et al. Telomere biology in neuroblastoma: telomere binding proteins and alternative lengthening of telomeres. J. Pediatr. Surg. 44, 2258–2266 (2009).
Bower, K. et al. Loss of wild-type ATRX expression in somatic cell hybrids segregates with activation of alternative lengthening of telomeres. PLoS ONE 7, e50062 (2012).
Capasso, M. et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nature Genet. 41, 718–723 (2009).
Wang, K. et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature 469, 216–220 (2011).
Nguyen le, B. et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility loci. PLoS Genet. 7, e1002026 (2011).
Diskin, S. J. et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nature Genet. 44, 1126–1130 (2012).
Maris, J. M. et al. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N. Engl. J. Med. 358, 2585–2593 (2008). References 52–56 showed an association of SNPs with neuroblastoma on the basis of genome-wide association studies.
Latorre, V. et al. Replication of neuroblastoma SNP association at the BARD1 locus in African-Americans. Cancer Epidemiol. Biomarkers Prev. 21, 658–663 (2012).
Bosse, K. R. et al. Common variation at BARD1 results in the expression of an oncogenic isoform that influences neuroblastoma susceptibility and oncogenicity. Cancer Res. 72, 2068–2078 (2012).
Molenaar, J. J. et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nature Genet. 44, 1199–1206 (2012).
Shimada, H. et al. The International Neuroblastoma Pathology Classification (the Shimada system). Cancer 86, 364–372 (1999). A description of the widely used Shimada neuroblastoma classification system.
Sidell, N. Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J. Natl Cancer Inst. 68, 589–596 (1982).
Matthay, K. K. et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children's oncology group study. J. Clin. Oncol. 27, 1007–1013 (2009). A follow up of the first randomized trial to demonstrate the benefit of autologous bone marrow transplantation and 13- cis -retinoic acid in high-risk neuroblastoma.
Acosta, S. et al. Identification of tumoral glial precursor cells in neuroblastoma. Cancer Lett. 312, 73–81 (2011).
Yanagisawa, M., Yoshimura, S. & Yu, R. K. Expression of GD2 and GD3 gangliosides in human embryonic neural stem cells. ASN Neuro. 3, e00054 (2011).
Shochat, S. J., Abt, A. B. & Schengrund, C. L. VCN-releasable sialic acid and gangliosides in human neuroblastomas. J. Pediatr. Surg. 12, 413–418 (1977).
Schulz, G. et al. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 44, 5914–5920 (1984).
Eisenhofer, G., Kopin, I. J. & Goldstein, D. S. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol. Rev. 56, 331–349 (2004).
Sisson, J. C. & Yanik, G. A. Theranostics: evolution of the radiopharmaceutical meta-iodobenzylguanidine in endocrine tumors. Semin. Nucl. Med. 42, 171–184 (2012).
Matthay, K. K., George, R. E. & Yu, A. L. Promising therapeutic targets in neuroblastoma. Clin. Cancer Res. 18, 2740–2753 (2012).
Eisenhofer, G., Kopin, I. J. & Goldstein, D. S. Leaky catecholamine stores: undue waste or a stress response coping mechanism? Ann. NY Acad. Sci. 1018, 224–230 (2004).
Schuldiner, S., Shirvan, A. & Linial, M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol. Rev. 75, 369–392 (1995).
Saier, M. H. Jr & Paulsen, I. T. Phylogeny of multidrug transporters. Semin. Cell Dev. Biol. 12, 205–213 (2001).
Hiyoshi, H. et al. Quiescence and γH2AX in neuroblastoma are regulated by ouabain/Na, K-ATPase. Br. J. Cancer 106, 1807–1815 (2012).
Norris, M. D. et al. Expression of the gene for multidrug-resistance-associated protein and outcome in patients with neuroblastoma. N. Engl. J. Med. 334, 231–238 (1996).
Henderson, M. J. et al. ABCC multidrug transporters in childhood neuroblastoma: clinical and biological effects independent of cytotoxic drug efflux. J. Natl Cancer Inst. 103, 1236–1251 (2011).
Decock, A., Ongenaert, M., Vandesompele, J. & Speleman, F. Neuroblastoma epigenetics: from candidate gene approaches to genome-wide screenings. Epigenetics 6, 962–970 (2011).
Marusyk, A., Almendro, V. & Polyak, K. Intra-tumour heterogeneity: a looking glass for cancer? Nature Rev. Cancer 12, 323–334 (2012).
Stallings, R. L., Foley, N. H., Bryan, K., Buckley, P. G. & Bray, I. Therapeutic targeting of miRNAs in neuroblastoma. Expert Opin. Ther. Targets 14, 951–962 (2010).
Speleman, F., De Preter, K. & Vandesompele, J. Neuroblastoma genetics and phenotype: a tale of heterogeneity. Semin. Cancer Biol. 21, 238–244 (2011).
Mestdagh, P. et al. An integrative genomics screen uncovers ncRNA T-UCR functions in neuroblastoma tumours. Oncogene 29, 3583–3592 (2010).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Benard, J. et al. MYCN-non-amplified metastatic neuroblastoma with good prognosis and spontaneous regression: a molecular portrait of stage 4S. Mol. Oncol. 2, 261–271 (2008).
Look, A. T., Hayes, F. A., Nitschke, R., McWilliams, N. B. & Green, A. A. Cellular DNA content as a predictor of response to chemotherapy in infants with unresectable neuroblastoma. N. Engl. J. Med. 311, 231–235 (1984). The first description of a relationship between chromosomal ploidy and outcome for neuroblastoma.
Schleiermacher, G. et al. Accumulation of segmental alterations determines progression in neuroblastoma. J. Clin. Oncol. 28, 3122–3130 (2010).
Caron, H. et al. Allelic loss of chromosome 1p as a predictor of unfavorable outcome in patients with neuroblastoma. N. Engl. J. Med. 334, 225–230 (1996).
Attiyeh, E. F. et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N. Engl. J. Med. 353, 2243–2253 (2005).
Bown, N. et al. Gain of chromosome arm 17q and adverse outcome in patients with neuroblastoma. N. Engl. J. Med. 340, 1954–1961 (1999).
Vandesompele, J. et al. Unequivocal delineation of clinicogenetic subgroups and development of a new model for improved outcome prediction in neuroblastoma. J. Clin. Oncol. 23, 2280–2299 (2005).
Stephens, P. J. et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144, 27–40 (2011).
Raffaghello, L. et al. Mechanisms of immune evasion of human neuroblastoma. Cancer Lett. 228, 155–161 (2005).
Coughlin, C. M. et al. Immunosurveillance and survivin-specific T-cell immunity in children with high-risk neuroblastoma. J. Clin. Oncol. 24, 5725–5734 (2006).
Favrot, M. C. et al. Expression of leucocyte adhesion molecules on 66 clinical neuroblastoma specimens. Int. J. Cancer 48, 502–510 (1991).
Foreman, N. K., Rill, D. R., Coustan-Smith, E., Douglass, E. C. & Brenner, M. K. Mechanisms of selective killing of neuroblastoma cells by natural killer cells and lymphokine activated killer cells. Potential for residual disease eradication. Br. J. Cancer 67, 933–938 (1993).
Castriconi, R. et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc. Natl Acad. Sci. USA 101, 12640–12645 (2004).
Raffaghello, L. et al. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia 6, 558–568 (2004).
Morandi, F. et al. Bone marrow-infiltrating human neuroblastoma cells express high levels of calprotectin and HLA-G proteins. PLoS ONE 7, e29922 (2012).
Asgharzadeh, S. et al. Prognostic significance of gene expression profiles of metastatic neuroblastomas lacking MYCN gene amplification. J. Natl Cancer Inst. 98, 1193–1203 (2006).
Song, L. et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J. Clin. Invest. 119, 1524–1536 (2009). The first demonstration of the importance of NKT cells in modulating TAMs in neuroblastoma.
Shurin, G. V., Gerein, V., Lotze, M. T. & Barksdale, E. M. Jr. Apoptosis induced in T cells by human neuroblastoma cells: role of Fas ligand. Nature Immun. 16, 263–274 (1998).
Liu, D. et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J. Clin. Invest. 122, 2221–2233 (2012).
Dong, L., Liu, Y., Colberg-Poley, A. M., Kaucic, K. & Ladisch, S. Induction of GM1a/GD1b synthase triggers complex ganglioside expression and alters neuroblastoma cell behavior; a new tumor cell model of ganglioside function. Glycoconj. J. 28, 137–147 (2011).
Ragupathi, G. et al. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin. Cancer Res. 9, 5214–5220 (2003).
Shurin, G. V. et al. Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 61, 363–369 (2001).
Ollert, M. W. et al. Normal human serum contains a natural IgM antibody cytotoxic for human neuroblastoma cells. Proc. Natl Acad. Sci. USA 93, 4498–4503 (1996).
Cheung, N. K. et al. Decay-accelerating factor protects human tumor cells from complement-mediated cytotoxicity in vitro. J. Clin. Invest. 81, 1122–1128 (1988).
Chen, S., Caragine, T., Cheung, N. K. & Tomlinson, S. CD59 expressed on a tumor cell surface modulates decay-accelerating factor expression and enhances tumor growth in a rat model of human neuroblastoma. Cancer Res. 60, 3013–3018 (2000).
Tarek, N. et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J. Clin. Invest. 122, 3260–3270 (2012). The first description of the mechanism of unlicensed NK cells during anti-GD2 MAb therapy of neuroblastoma.
Cheung, N. K. et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J. Clin. Oncol. 30, 3264–3270 (2012). A detailed clinical summary of two decades of anti-GD2 MAb 3F8 therapy of neuroblastoma and the prognostic importance of bone marrow minimal residual disease measurement using quantitative reverse transcription PCR during adjuvant immunotherapy.
Mellman, I., Coukos, G. & Dranoff, G. Cancer immunotherapy comes of age. Nature 480, 480–489 (2011).
Cheever, M. A. et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 15, 5323–5337 (2009).
Bao, L., Dunham, K. & Lucas, K. MAGE-A1, MAGE-A3, and NY-ESO-1 can be upregulated on neuroblastoma cells to facilitate cytotoxic T lymphocyte-mediated tumor cell killing. Cancer Immunol. Immunother. 60, 1299–1307 (2011).
Sarkar, A. K. & Nuchtern, J. G. Lysis of MYCN-amplified neuroblastoma cells by MYCN peptide-specific cytotoxic T lymphocytes. Cancer Res. 60, 1908–1913 (2000).
Fest, S. et al. Survivin minigene DNA vaccination is effective against neuroblastoma. Int. J. Cancer 125, 104–114 (2009).
Jing, W., Yan, X., Hallett, W. H., Gershan, J. A. & Johnson, B. D. Depletion of CD25+ T cells from hematopoietic stem cell grafts increases posttransplantation vaccine-induced immunity to neuroblastoma. Blood 117, 6952–6962 (2011).
Louis, C. U. & Brenner, M. K. Cellular immunotherapy for neuroblastoma: a review of current vaccine and adoptive T cell therapeutics. Curr. Pharm. Des. 15, 424–429 (2009).
Caruso, D. A. et al. Results of a Phase I study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children with Stage 4 neuroblastoma. Cancer 103, 1280–1291 (2005).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nature Rev. Cancer 12, 252–264 (2012).
Kramer, K. et al. Disialoganglioside G(D2) loss following monoclonal antibody therapy is rare in neuroblastoma. Clin. Cancer Res. 4, 2135–2139 (1998).
Yu, A. L. et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 363, 1324–1334 (2010). The first randomized study to show clinical efficacy of an anti-GD2 MAb in neuroblastoma.
Cheung, N. K. et al. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J. Clin. Oncol. 5, 1430–1440 (1987). The first in-human use of anti-GD2 MAb 3F8 in a Phase I trial in neuroblastoma showing pain as the major side effect.
Cheung, I. Y., Hsu, K. & Cheung, N. K. Activation of peripheral-blood granulocytes is strongly correlated with patient outcome after immunotherapy with Anti-GD2 monoclonal antibody and granulocyte-macrophage colony-stimulating factor. J. Clin. Oncol. 30, 426–432 (2012). The first demonstration of granulocyte activation and clinical outcome during anti-GD2 MAb therapy of neuroblastoma.
Leung, W. Use of NK cell activity in cure by transplant. Br. J. Haematol. 155, 14–29 (2011).
Venstrom, J. M. et al. KIR and HLA genotypes are associated with disease progression and survival following autologous hematopoietic stem cell transplantation for high-risk neuroblastoma. Clin. Cancer Res. 15, 7330–7334 (2009).
Delgado, D. C. et al. Genotypes of NK cell KIR receptors, their ligands, and Fcγ receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res. 70, 9554–9561 (2010). A demonstration of the association of clinical response to KIR and FcγR polymorphism in immunocytokine therapy of neuroblastoma.
Metelitsa, L. S. et al. Antidisialoganglioside/granulocyte macrophage-colony-stimulating factor fusion protein facilitates neutrophil antibody-dependent cellular cytotoxicity and depends on FcγRII (CD32) and Mac-1 (CD11b/CD18) for enhanced effector cell adhesion and azurophil granule exocytosis. Blood 99, 4166–4173 (2002).
Kushner, B. H. & Cheung, N. K. Clinically effective monoclonal antibody 3F8 mediates nonoxidative lysis of human neuroectodermal tumor cells by polymorphonuclear leukocytes. Cancer Res. 51, 4865–4870 (1991).
Kushner, B. H. & Cheung, N. K. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood 73, 1936–1941 (1989).
Kushner, B. H. & Cheung, N. K. Absolute requirement of CD11/CD18 adhesion molecules, FcRII and the phosphatidylinositol-linked FcRIII for monoclonal antibody-mediated neutrophil antihuman tumor cytotoxicity. Blood 79, 1484–1490 (1992).
Cheung, N. K. et al. FCGR2A polymorphism is correlated with clinical outcome after immunotherapy of neuroblastoma with anti-GD2 antibody and granulocyte macrophage colony-stimulating factor. J. Clin. Oncol. 24, 2885–2890 (2006). The first demonstration of the importance of FCGR2A polymorphism during anti-GD2 MAb therapy of neuroblastoma.
Ross, G. D., Vetvicka, V., Yan, J., Xia, Y. & Vetvickova, J. Therapeutic intervention with complement and β-glucan in cancer. Immunopharmacology 42, 61–74 (1999).
Diamond, M. S. & Springer, T. A. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J. Cell Biol. 120, 545–556 (1993).
Cheung, N. K. & Modak, S. Oral (1→3),(1→4)-β-d-glucan synergizes with antiganglioside GD2 monoclonal antibody 3F8 in the therapy of neuroblastoma. Clin. Cancer Res. 8, 1217–1223 (2002).
Asgharzadeh, S. et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J. Clin. Oncol. 30, 3525–3532 (2012).
Ren, Y. et al. Inhibition of tumor growth and metastasis in vitro and in vivo by targeting macrophage migration inhibitory factor in human neuroblastoma. Oncogene 25, 3501–3508 (2006).
Ren, Y. et al. Upregulation of macrophage migration inhibitory factor contributes to induced N-Myc expression by the activation of ERK signaling pathway and increased expression of interleukin-8 and VEGF in neuroblastoma. Oncogene 23, 4146–4154 (2004).
Biswas, S. K. & Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature Immunol. 11, 889–896 (2010).
Munn, D. H. & Cheung, N. K. Antibody-dependent antitumor cytotoxicity by human monocytes cultured with recombinant macrophage colony-stimulating factor. Induction of efficient antibody-mediated antitumor cytotoxicity not detected by isotope release assays. J. Exp. Med. 170, 511–526 (1989).
Petrella, T. et al. Single-agent interleukin-2 in the treatment of metastatic melanoma: a systematic review. Cancer Treat. Rev. 33, 484–496 (2007).
Ladenstein, R. et al. Dose finding study for the use of subcutaneous recombinant interleukin-2 to augment natural killer cell numbers in an outpatient setting for stage 4 neuroblastoma after megatherapy and autologous stem-cell reinfusion. J. Clin. Oncol. 29, 441–448 (2011).
Steel, J. C., Waldmann, T. A. & Morris, J. C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 33, 35–41 (2012).
Berger, C. et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood 114, 2417–2426 (2009).
Vivier, E., Ugolini, S., Blaise, D., Chabannon, C. & Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nature Rev. Immunol. 12, 239–252 (2012).
Metelitsa, L. S. et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J. Exp. Med. 199, 1213–1221 (2004).
Neal, Z. C. et al. Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin. Cancer Res. 10, 4839–4847 (2004).
Shusterman, S. et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J. Clin. Oncol. 28, 4969–4975 (2010).
Yang, R. K. et al. Intratumoral hu14.18-IL-2 (IC) induces local and systemic antitumor effects that involve both activated T and NK cells as well as enhanced IC retention. J. Immunol. 189, 2656–2664 (2012).
Becker, J. C., Varki, N., Gillies, S. D., Furukawa, K. & Reisfeld, R. A. Long-lived and transferable tumor immunity in mice after targeted interleukin-2 therapy. J. Clin. Invest. 98, 2801–2804 (1996).
Albertini, M. R. et al. Phase II trial of hu14.18-IL2 for patients with metastatic melanoma. Cancer Immunol. Immunother. 61, 2261–2271 (2012).
Cheung, N. K., Guo, H. F., Heller, G. & Cheung, I. Y. Induction of Ab3 and Ab3' antibody was associated with long-term survival after anti-G(D2) antibody therapy of stage 4 neuroblastoma. Clin. Cancer Res. 6, 2653–2660 (2000). The first demonstration of an anti-idiotype network after anti-GD2 MAb therapy in neuroblastoma.
Foon, K. A. et al. Clinical and immune responses in advanced melanoma patients immunized with an anti-idiotype antibody mimicking disialoganglioside GD2. J. Clin. Oncol. 18, 376–384 (2000).
Cheung, N. K., Canete, A., Cheung, I. Y., Ye, J. N. & Liu, C. Disialoganglioside GD2 anti-idiotypic monoclonal antibodies. Int. J. Cancer 54, 499–505 (1993).
Bleeke, M. et al. Systematic amino acid substitutions improved efficiency of GD2-peptide mimotope vaccination against neuroblastoma. Eur. J. Cancer 45, 2915–2921 (2009).
Bolesta, E. et al. DNA vaccine expressing the mimotope of GD2 ganglioside induces protective GD2 cross-reactive antibody responses. Cancer Res. 65, 3410–3418 (2005).
Navid, F., Santana, V. M. & Barfield, R. C. Anti-GD2 antibody therapy for GD2-expressing tumors. Curr. Cancer Drug Targets 10, 200–209.
Kushner, B. H., Kramer, K., Modak, S. & Cheung, N. K. Successful multifold dose escalation of anti-GD2 monoclonal antibody 3F8 in patients with neuroblastoma: a phase I study. J. Clin. Oncol. 29, 1168–1174 (2011).
Cheung, N. K., Guo, H., Hu, J., Tassev, D. V. & Cheung, I. Y. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. Oncoimmunology 1, 477–486 (2012).
Carpenter, E. L. et al. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene 31, 4859–4867 (2012).
Novak-Hofer, I. The L1 cell adhesion molecule as a target for radioimmunotherapy. Cancer Biother. Radiopharm. 22, 175–184 (2007).
Modak, S., Kramer, K., Gultekin, S. H., Guo, H. F. & Cheung, N. K. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 61, 4048–4054 (2001).
Kramer, K. et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J. Neurooncol. 97, 409–418 (2010). The first in-human intrathecal (intra-ommaya) injection of radioiodinated anti-B7H3 MAb 8H9 as compartmental radioimmunotherapy to prolong survival following recurrent metastatic neuroblastoma to the brain.
Park, J. R. et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 15, 825–833 (2007).
Pule, M. A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature Med. 14, 1264–1270 (2008). The first demonstration of CAR-engineered T cells providing clinical benefit for neuroblastoma.
Craddock, J. A. et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 33, 780–788 (2010).
Sun, J. et al. T cells expressing constitutively active Akt resist multiple tumor-associated inhibitory mechanisms. Mol. Ther. 18, 2006–2017 (2010).
Lee, D. W., Barrett, D. M., Mackall, C., Orentas, R. & Grupp, S. A. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin. Cancer Res. 18, 2780–2790 (2012).
Cho, D. et al. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin. Cancer Res. 16, 3901–3909 (2010).
Denman, C. J. et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 7, e30264 (2012).
Yankelevich, M. et al. Anti-CD3 x anti-GD2 bispecific antibody redirects T-cell cytolytic activity to neuroblastoma targets. Pediatr. Blood Cancer 59, 1198–1205 (2012).
Cheung, N. V. & Heller, G. Chemotherapy dose intensity correlates strongly with response, median survival, and median progression-free survival in metastatic neuroblastoma. J. Clin. Oncol. 9, 1050–1058 (1991). The first retrospective analysis to demonstrate the effect of dose intensity of induction chemotherapy on neuroblastoma outcome.
Pearson, A. D. et al. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncol. 9, 247–256 (2008). The first prospective analysis to test the effect of dose intensity of induction chemotherapy in treating neuroblastoma on outcome.
Whiteford, C. C. et al. Credentialing preclinical pediatric xenograft models using gene expression and tissue microarray analysis. Cancer Res. 67, 32–40 (2007).
Bergman, I., Basse, P. H., Barmada, M. A., Griffin, J. A. & Cheung, N. K. Comparison of in vitro antibody-targeted cytotoxicity using mouse, rat and human effectors. Cancer Immunol. Immunother. 49, 259–266 (2000).
Shultz, L. D., Ishikawa, F. & Greiner, D. L. Humanized mice in translational biomedical research. Nature Rev. Immunol. 7, 118–130 (2007).
Pek, E. A., Chan, T., Reid, S. & Ashkar, A. A. Characterization and IL-15 dependence of NK cells in humanized mice. Immunobiology 216, 218–224 (2011).
Chesler, L. & Weiss, W. A. Genetically engineered murine models—contribution to our understanding of the genetics, molecular pathology and therapeutic targeting of neuroblastoma. Semin. Cancer Biol. 21, 245–255 (2011).
Quigley, D. & Balmain, A. Systems genetics analysis of cancer susceptibility: from mouse models to humans. Nature Rev. Genet. 10, 651–657 (2009).
Zhang, J. et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 481, 329–334 (2012).
Atkinson, J. M. et al. An integrated in vitro and in vivo high-throughput screen identifies treatment leads for ependymoma. Cancer Cell 20, 384–399 (2011).
Huber, K. et al. Persistent expression of BMP-4 in embryonic chick adrenal cortical cells and its role in chromaffin cell development. Neural Dev. 3, 28 (2008).
Betters, E., Liu, Y., Kjaeldgaard, A., Sundstrom, E. & Garcia-Castro, M. I. Analysis of early human neural crest development. Dev. Biol. 344, 578–592 (2010).
Lau, L., Dagg, R., Haber, M., Murray, J. & Reddel, R. R. Alternative Lengthening of Telomeres (ALT) in neuroblastoma tumors. Adv. Neuroblastoma Res. Abstr. (2012).
Scott, A. M., Wolchok, J. D. & Old, L. J. Antibody therapy of cancer. Nature Rev. Cancer 12, 278–287 (2012).
Brodeur, G. M. et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 11, 1466–1477 (1993).
Conte, M. et al. Neuroblastoma in adolescents: the Italian experience. Cancer 106, 1409–1417 (2006).
Brignole, C. et al. Effect of bortezomib on human neuroblastoma cell growth, apoptosis, and angiogenesis. J. Natl Cancer Inst. 98, 1142–1157 (2006).
Fulda, S. The PI3K/Akt/mTOR pathway as therapeutic target in neuroblastoma. Curr. Cancer Drug Targets 9, 729–737 (2009).
Carol, H. et al. Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother. Pharmacol. 68, 1291–1304 (2011).
Molenaar, J. J. et al. Cyclin D1 and CDK4 activity contribute to the undifferentiated phenotype in neuroblastoma. Cancer Res. 68, 2599–2609 (2008).
Yee, D. et al. Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation. A potential autocrine growth factor. J. Clin. Invest. 86, 1806–1814 (1990).
Tanno, B. et al. Down-regulation of insulin-like growth factor I receptor activity by NVP-AEW541 has an antitumor effect on neuroblastoma cells in vitro and in vivo. Clin. Cancer Res. 12, 6772–6780 (2006).
Brodeur, G. M. et al. Trk receptor expression and inhibition in neuroblastomas. Clin. Cancer Res. 15, 3244–3250 (2009).
Goldsmith, K. C. et al. Mitochondrial Bcl-2 family dynamics define therapy response and resistance in neuroblastoma. Cancer Res. 72, 2565–2577 (2012).
Petroni, M., Veschi, V., Gulino, A. & Giannini, G. Molecular mechanisms of MYCN-dependent apoptosis and the MDM2-p53 pathway: an Achille's heel to be exploited for the therapy of MYCN-amplified neuroblastoma. Front. Oncol. 2, 141 (2012).
Coffey, D. C. et al. The histone deacetylase inhibitor, CBHA, inhibits growth of human neuroblastoma xenografts in vivo, alone and synergistically with all-trans retinoic acid. Cancer Res. 61, 3591–3594 (2001).
Pietras, A., Johnsson, A. S. & Pahlman, S. The HIF-2α-driven pseudo-hypoxic phenotype in tumor aggressiveness, differentiation, and vascularization. Curr. Top. Microbiol. Immunol. 345, 1–20 (2010).
Crosswell, H. E. et al. PHA665752, a small-molecule inhibitor of c-Met, inhibits hepatocyte growth factor-stimulated migration and proliferation of c-Met-positive neuroblastoma cells. BMC Cancer 9, 411 (2009).
Sugiura, Y., Shimada, H., Seeger, R. C., Laug, W. E. & DeClerck, Y. A. Matrix metalloproteinases-2 and -9 are expressed in human neuroblastoma: contribution of stromal cells to their production and correlation with metastasis. Cancer Res. 58, 2209–2216 (1998).
Xu, H., Cheung, I. Y., Guo, H. F. & Cheung, N. K. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 69, 6275–6281 (2009).
Reid, G. S. et al. Interferon-γ-dependent infiltration of human T cells into neuroblastoma tumors in vivo. Clin. Cancer Res. 15, 6602–6608 (2009).
Levy, A. G. et al. The combination of the novel glycolysis inhibitor 3-BrOP and rapamycin is effective against neuroblastoma. Invest. New Drugs 30, 191–199 (2012).
Teitz, T. et al. Caspase-8 deficiency enhances neuroblastoma metastasis in vivo — a new mouse model for metastatic neuroblastoma. Advances in Neuroblastoma Research Abstr. (2012).
Chanthery, Y. H. et al. Paracrine signaling through MYCN enhances tumor-vascular interactions in neuroblastoma. Sci. Transl. Med. 4, 115ra3 (2012).
Teitz, T. et al. Preclinical models for neuroblastoma: establishing a baseline for treatment. PLoS ONE 6, e19133 (2011).
Huebener, N. et al. Xenogeneic immunization with human tyrosine hydroxylase DNA vaccines suppresses growth of established neuroblastoma. Mol. Cancer Ther. 8, 2392–2401 (2009).
Ahmed, M., Goldgur, Y., Hu, J., Guo, H.F. & Cheung, N. K. V. In silico driven redesign of a clinically relevant antibody for the treatment of GD2-positive tumors. PLoS ONE (in the press).
Gao, J. et al. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 71, 1029–1040 (2011).
Zhao, Q., Feng, Y., Zhu, Z. & Dimitrov, D. S. Human monoclonal antibody fragments binding to insulin-like growth factors I and II with picomolar affinity. Mol. Cancer Ther. 10, 1677–1685 (2011).
Acknowledgements
The authors thank I. Cheung and B. Kushner for their critical review of this manuscript. This work was supported in part by grants from the US National Cancer Institute (NCI) and US Department of Defense (DOD) (NCI-CA161978, DOD-PR111043 and NCI-CA154754 (to N.K.C.)), as well as grants from the US National Institutes of Health (NIH) and NCI (NCI-CA21765, NIH-EY014867, NIH-EY018599 and NCI-CA168875), and funding from the American Lebanese Syrian Associated Charities and the Howard Hughes Medical Institute Early Career Scientist Award (to M.A.D.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Memorial Sloan-Kettering Cancer Center (MSKCC) has a patent application on hu3F8 and N.K.C. was named as one of the inventors. MSKCC has licensed the patent on β-glucan to Biotec Pharmacon, and the patent on antibody 8H9 to United Therapeutics, and N.K.C. was named as one of the inventors for both agents.
Related links
Glossary
- GD2
-
A disialoganglioside expressed on tumours of neuroectodermal origin, including human neuroblastoma, melanoma, small-cell lung cancer and many sarcomas, with highly restricted expression on normal tissues (such as the cerebellum and peripheral nerves). Two monoclonal antibody families specific for the oligosaccharide epitope of GD2 have been tested extensively in patients, namely the mouse immunoglobulin G3 (IgG3) antibody 3F8 and its humanized version (hu3F8), and the mouse IgG2a antibody 14G2a and its chimeric (ch14.18) or humanized (hu14.18) forms.
- Myeloablative
-
Bone marrow ablation owing to the loss of haematopoietic stem cells following high-dose radiation or chemotherapy.
- Adrenal gland
-
Endocrine organ responsible for generating stress hormones, aldosterone and androgens.
- Sympathetic ganglia
-
Masses of nerve cells that are part of a network controlling autonomic 'fight-or-flight' responses.
- Congenital central hypoventilation syndrome
-
(CCHS). A congenital brain stem disease in which autonomic control of breathing is defective, resulting in sleep apnoea.
- Hirschsprung disease
-
(HSCR). A congenital disease in which the large intestine lacks innervation.
- Chromaffin cells
-
Neuroendocrine cells in the adrenal medulla that receive sympathetic input and release catecholamine neurotransmitters to the systemic circulation.
- Event-free survival
-
A measure of time spent alive without a life-threatening adverse event.
- Alternative lengthening of telomeres
-
(ALT). A recombination-based mechanism that allows telomere length maintenance in the absence of telomerase activity.
- Schwannian stromal content
-
Glial cells in the surrounding stroma, interspersed with neuroblastoma cells. Tumours that are highly differentiated usually have a high Schwannian stromal content.
- Mitotic-karyorrhexis index
-
A measure of the frequency of cells in mitosis with karyorrhexis (nuclear fragmentation associated with cell death).
- Hyperdiploidy
-
Having more than the diploid number of chromosomes, where the DNA index is >1.15.
- Complement decay accelerating factor
-
(CD55). A 70 kDa membrane protein that prevents the assembly of the C3bBb complex (C3-convertase of the alternative pathway of complement activation), thereby blocking formation of the membrane attack complex (MAC).
- Protectin
-
(CD59). A membrane protein that inhibits the membrane attack complex (MAC) by binding C5b678 and preventing C9 from binding and polymerizing.
- Complement-mediated cytotoxicity
-
(CMC). Lysis of a cell resulting from triggering of the complement cascade, in which the binding of an immunoglobulin M (IgM) or IgG antibody to the cell surface is followed by the binding of complement proteins to that antibody.
- NK cell-mediated antibody-dependent cell-mediated cytotoxicity
-
(NK-ADCC). Killing of tumour cells by natural killer cells, the Fc receptor of which adheres to the antibody already attached to the target cell.
- Granulocyte ADCC
-
Killing of tumour cells by granulocytes, the Fc receptor of which adheres to the antibody already attached to the target cell.
- Killer cell immunoglobulin-like receptors
-
(KIRs). Highly polymorphic natural killer cell surface proteins that interact with major histocompatibility complex class I molecules. Most KIRs mediate natural killer cell inhibition instead of activation.
- β-glucan
-
Polysaccharides of D-glucose monomers linked by β-glycosidic bonds. β-glucan (1,3/1,4 linkages) from cereals such as barley or β-glucan (1,3/1,6 linkages) from mushroom and yeasts bind to the dectin 1 receptor and complement receptor 3 (CR3 or CD11b/CD18) and enhance receptor-mediated antitumour properties.
- Regulatory T cells
-
(TReg cells). A T cell subtype that releases suppressive cytokines and silences immune responses.
- Anti-idiotype
-
An antibody binding specifically to an epitope in the variable region of another antibody; when the epitope is the actual antigen-binding site, an anti-idiotypic antibody can mimic the de novo antigen.
Rights and permissions
About this article
Cite this article
Cheung, NK., Dyer, M. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer 13, 397–411 (2013). https://doi.org/10.1038/nrc3526
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3526
This article is cited by
-
Predictive value of 18 F-FDG PET/CT versus bone marrow biopsy and aspiration in pediatric neuroblastoma
Clinical & Experimental Metastasis (2024)
-
Anaplastic lymphoma kinase overexpression enhances aggressive phenotypic characteristics of endometrial carcinoma
BMC Cancer (2023)
-
β3-adrenergic receptor on tumor-infiltrating lymphocytes sustains IFN-γ-dependent PD-L1 expression and impairs anti-tumor immunity in neuroblastoma
Cancer Gene Therapy (2023)
-
Targeting GD2 after allogeneic SCT: effector cell composition defines the optimal use of ch14.18 and the bispecific antibody construct NG-CU (GD2-CD3)
Cancer Immunology, Immunotherapy (2023)
-
Synergistic anti-proliferative and apoptotic effect of NVP-BEZ235 and curcumin on human SH-SY5Y neuroblastoma cells
Medical Oncology (2023)