Key Points
-
The core RNA polymerase (Pol) subunits and general transcription factors (GTFs) are rarely mutated in cancer, although some GTFs are consistently overexpressed in tumours and this is thought to contribute to malignancy in some cases.
-
Subunits of the mediator complex are increasingly being found to be mutated or amplified in tumours, where they have oncogenic or tumour suppressive activities and functions, depending on the genetic background and cellular context.
-
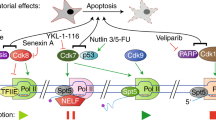
Regulators of post-initiation stages of transcription, particularly components of RNA Pol II super elongation complexes (SECs), are recurrently mutated in cancer, particularly haematological malignancies through translocation with the mixed lineage leukaemia (MLL) family of transcription factors. The resultant fusion proteins facilitate the enhanced transcription elongation of homeobox (HOX) transcription factors that are involved in embryonic development and haematopoietic cell differentiation, which drives malignancy.
-
RNA Pol I and RNA Pol III are consistently dysregulated in cancer, which is mostly mediated through upstream oncogenic and tumour suppressive signalling pathways rather than through mutations.
-
The most potent and pervasive oncogenic and tumour suppressive components of the transcription apparatus seem to be those that are capable of modulating all three RNA Pols.
-
RNA Pol I transcriptional overactivity has been shown to be necessary for the survival of haematological tumour cells, and the RNA Pol I GTF SL-1 has been successfully targeted using a small-molecule inhibitor to therapeutically treat transgenic mouse models of cancer in vivo. RNA Pol I transcription therapy is currently entering Phase I trials in humans for the treatment of lymphoma and leukaemia.
-
Components of the core transcription apparatus, including the mediator complex and the SEC, represent bone fide therapeutic targets for cancer treatment not only as advanced broad-spectrum cytotoxics but also potentially as part of the new paradigm of personalized medicine.
Abstract
Mutations that directly affect transcription by RNA polymerases rank among the most central mediators of malignant transformation, but the frequency of new anticancer drugs that selectively target defective transcription apparatus entering the clinic has been limited. This is because targeting the large protein–protein and protein–DNA interfaces that control both generic and selective aspects of RNA polymerase transcription has proved extremely difficult. However, recent technological advances have led to a 'quantum leap' in our comprehension of the structure and function of the core RNA polymerase components, how they are dysregulated in a broad range of cancers and how they may be targeted for 'transcription therapy'.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Roeder, R. G. & Rutter, W. J. Multiple Forms of DNA-Dependent Rna Polymerase in Eukaryotic Organisms. Nature 224, 234–237 (1969). A seminal paper in the RNA Pol transcription field; describes the first isolation of RNA Pol I, Pol II and Pol III from developing sea urchin embryos and rat liver.
Weinmann, R., Raskas, H. J. & Roeder, R. G. Role of DNA-Dependent Rna Polymerase-Ii and Polymerase-Iii in Transcription of Adenovirus Genome Late in Productive Infection. Proc. Natl Acad. Sci. USA 71, 3426–3430 (1974).
Weinmann, R. & Roeder, R. G. Role of DNA-Dependent Rna-Polymerase Iii in Transcription of Transfer-Rna and 5s Rna Genes. Proc. Natl Acad. Sci. USA 71, 1790–1794 (1974).
Guttman, M. & Rinn, J. L. Modular regulatory principles of large non-coding RNAs. Nature 482, 339–346 (2012).
Esteller, M. Non-coding RNAs in human disease. Nature Rev. Genet. 12, 861–874 (2011).
Alexander, R. P., Fang, G., Rozowsky, J., Snyder, M. & Gerstein, M. B. Annotating non-coding regions of the genome. Nature Rev. Genet. 11, 559–571 (2010).
Mercer, T. R., Dinger, M. E. & Mattick, J. S. Long non-coding RNAs: insights into functions. Nature Rev. Genet. 10, 155–159 (2009).
He, L. & Hannon, G. J. MicroRNAs: small RNAs with a big role in gene regulation. Nature Rev. Genet. 5, 522–531 (2004).
White, R. J. Transcription by RNA polymerase III: more complex than we thought. Nature Rev. Genet. 12, 459–463 (2011).
Noma, K. & Kamakaka, R. T. The human Pol III transcriptome and gene information flow. Nature Struct. Mol. Biol. 17, 539–541 (2010).
Dieci, G., Fiorino, G., Castelnuovo, M., Teichmann, M. & Pagano, A. The expanding RNA polymerase III transcriptome. Trends Genet. 23, 614–622 (2007).
Nikitina, T. V., Tischenko, L. I. & Schulz, W. A. Recent insights into regulation of transcription by RNA polymerase III and the cellular functions of its transcripts. Biol. Chem. 392, 395–404 (2011).
Sklar, V. E. F., Schwartz, L. B. & Roeder, R. G. Distinct Molecular-Structures of Nuclear Class I, Ii, and Iii DNA-Dependent Rna Polymerases. Proc. Natl Acad. Sci. USA 72, 348–352 (1975). One of the first papers to carry out a comparative structure analysis of the three RNA Pols.
Naidu, S., Friedrich, J. K., Russell, J. & Zomerdijk, J. C. TAF1B is a TFIIB-like component of the basal transcription machinery for RNA polymerase I. Science 333, 1640–1642 (2011).
Vannini, A. & Cramer, P. Conservation between the RNA Polymerase I, II, and III Transcription Initiation Machineries. Mol. Cell 45, 439–446 (2012). A more contemporary review of the structural conservation evident between all RNA Pols, suggesting that all Pols contain a conserved core that is involved in transcription initiation.
Cramer, P. et al. Structure of eukaryotic RNA polymerases. Annu. Rev. Biophys. 37, 337–352 (2008).
Sekine, S., Tagami, S. & Yokoyama, S. Structural basis of transcription by bacterial and eukaryotic RNA polymerases. Curr. Opin. Struct. Biol. 22, 110–118 (2012).
Werner, M., Thuriaux, P. & Soutourina, J. Structure-function analysis of RNA polymerases I and III. Curr. Opin. Struct. Biol. 19, 740–745 (2009).
Bywater, M. J. et al. Inhibition of RNA Polymerase I as a Therapeutic Strategy to Promote Cancer-Specific Activation of p53. Cancer Cell 22, 51–65 (2012). This paper provided the first evidence that cancer cells can be dependent on high rates of RNA Pol I transcription, and that this dependency can be targeted by a small-molecule inhibitor that selectively inhibits RNA Pol I transcription.
Roeder, R. G. The eukaryotic transcriptional machinery: complexities and mechanisms unforeseen. Nature Med. 9, 1239–1244 (2003). A comprehensive review of the initial discovery of RNA Pols, and the elucidation of their complex components.
Schmitz, K. M. et al. TAF12 recruits Gadd45a and the nucleotide excision repair complex to the promoter of rRNA genes leading to active DNA demethylation. Mol. Cell 33, 344–353 (2009).
Drygin, D. et al. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res. 71, 1418–1430 (2011). Details the development of the first selective inhibitor of RNA Pol I.
Compe, E. & Egly, J. M. TFIIH: when transcription met DNA repair. Nature Rev. Mol. Cell Biol. 13, 343–354 (2012).
Iben, S. et al. TFIIH plays an essential role in RNA polymerase I transcription. Cell 109, 297–306 (2002).
Bradsher, J. et al. CSB is a component of RNA pol I transcription. Mol. Cell 10, 819–829 (2002).
Assfalg, R. et al. TFIIH is an elongation factor of RNA polymerase I. Nucleic Acids Res. 40, 650–659 (2012).
Egly, J. M. & Coin, F. A history of TFIIH: two decades of molecular biology on a pivotal transcription/repair factor. DNA Repair (Amst.) 10, 714–721 (2011).
Wang, F. et al. DNA repair gene XPD polymorphisms and cancer risk: a meta-analysis based on 56 case-control studies. Cancer Epidemiol. Biomarkers Prev. 17, 507–517 (2008).
Manuguerra, M. et al. XRCC3 and XPD/ERCC2 single nucleotide polymorphisms and the risk of cancer: a HuGE review. Am. J. Epidemiol. 164, 297–302 (2006).
Xue, H. et al. The effect of XPD/ERCC2 polymorphisms on gastric cancer risk among different ethnicities: a systematic review and meta-analysis. PLoS ONE 7, e43431 (2012).
Johnson, S. A. et al. Increased expression of TATA-binding protein, the central transcription factor, can contribute to oncogenesis. Mol. Cell. Biol. 23, 3043–3051 (2003). The first study demonstrating that the overexpression of TBP alone can be transformative, and that TBP is overexpressed in some colon cancers.
Li, B. Q., Huang, T., Liu, L., Cai, Y. D. & Chou, K. C. Identification of colorectal cancer related genes with mRMR and shortest path in protein-protein interaction network. PLoS ONE 7, e33393 (2012).
Daly, N. L. et al. Deregulation of RNA polymerase III transcription in cervical epithelium in response to high-risk human papillomavirus. Oncogene 24, 880–888 (2005).
Lockwood, W. W. et al. Integrative genomic analyses identify BRF2 as a novel lineage-specific oncogene in lung squamous cell carcinoma. PLoS Med. 7, e1000315 (2010). The first study to describe the amplification of BRF2 in lung squamous cell carcinoma, and that overexpression of BRF2 alone can be transformative.
Zhong, S., Zhang, C. & Johnson, D. L. Epidermal growth factor enhances cellular TATA binding protein levels and induces RNA polymerase I- and III-dependent gene activity. Mol. Cell. Biol. 24, 5119–5129 (2004).
Colgan, J. & Manley, J. L. TFIID can be rate limiting in vivo for TATA-containing, but not TATA-lacking, RNA polymerase II promoters. Genes Dev. 6, 304–315 (1992).
Majello, B., Napolitano, G., De Luca, P. & Lania, L. Recruitment of human TBP selectively activates RNA polymerase II TATA-dependent promoters. J. Biol. Chem. 273, 16509–16516 (1998).
Johnson, S. A., Dubeau, L. & Johnson, D. L. Enhanced RNA polymerase III-dependent transcription is required for oncogenic transformation. J. Biol. Chem. 283, 19184–19191 (2008).
Trivedi, A., Vilalta, A., Gopalan, S. & Johnson, D. L. TATA-binding protein is limiting for both TATA-containing and TATA-lacking RNA polymerase III promoters in Drosophila cells. Mol. Cell. Biol. 16, 6909–6916 (1996).
Wang, H. D., Trivedi, A. & Johnson, D. L. Regulation of RNA polymerase I-dependent promoters by the hepatitis B virus X protein via activated Ras and TATA-binding protein. Mol. Cell. Biol. 18, 7086–7094 (1998).
Sadovsky, Y. et al. Transcriptional activators differ in their responses to overexpression of TATA-box-binding protein. Mol. Cell. Biol. 15, 1554–1563 (1995).
Dauwerse, J. G. et al. Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nature Genet. 43, 20–22 (2011).
Poortinga, G. et al. c-MYC coordinately regulates ribosomal gene chromatin remodeling and Pol I availability during granulocyte differentiation. Nucleic Acids Res. 39, 3267–3281 (2011). This paper describes the coordinate regulation of a cohort of RNA Pol I transcription apparatus components by MYC.
Winter, A. G. et al. RNA polymerase III transcription factor TFIIIC2 is overexpressed in ovarian tumors. Proc. Natl Acad. Sci. USA 97, 12619–12624 (2000).
Garcia, M. J. et al. A 1 Mb minimal amplicon at 8p11-12 in breast cancer identifies new candidate oncogenes. Oncogene 24, 5235–5245 (2005).
Melchor, L. et al. Genomic analysis of the 8p11-12 amplicon in familial breast cancer. Int. J. Cancer 120, 714–717 (2007).
Williams, S. V. et al. High-resolution analysis of genomic alteration on chromosome arm 8p in urothelial carcinoma. Genes Chromosomes Cancer 49, 642–659 (2010).
Cabarcas, S. & Schramm, L. RNA polymerase III transcription in cancer: the BRF2 connection. Mol. Cancer 10, 47 (2011).
Schramm, L. & Hernandez, N. Recruitment of RNA polymerase III to its target promoters. Genes Dev. 16, 2593–2620 (2002).
Huang, Y. & Maraia, R. J. Comparison of the RNA polymerase III transcription machinery in Schizosaccharomyces pombe, Saccharomyces cerevisiae and human. Nucleic Acids Res. 29, 2675–2690 (2001).
Geiduschek, E. P. & Kassavetis, G. A. The RNA polymerase III transcription apparatus. J. Mol. Biol. 310, 1–26 (2001).
Boeger, H. et al. Structural basis of eukaryotic gene transcription. FEBS Lett. 579, 899–903 (2005).
White, R. J. RNA polymerases I and III, non-coding RNAs and cancer. Trends Genet. 24, 622–629 (2008).
Kelleher, R. J., 3rd, Flanagan, P. M. & Kornberg, R. D. A novel mediator between activator proteins and the RNA polymerase II transcription apparatus. Cell 61, 1209–1215 (1990). The first paper to identify and describe the role of the mediator complex in yeast.
Kim, Y. J., Bjorklund, S., Li, Y., Sayre, M. H. & Kornberg, R. D. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell 77, 599–608 (1994).
Chao, D. M. et al. A mammalian SRB protein associated with an RNA polymerase II holoenzyme. Nature 380, 82–85 (1996).
Malik, S. & Roeder, R. G. Dynamic regulation of pol II transcription by the mammalian Mediator complex. Trends Biochem. Sci. 30, 256–263 (2005).
Conaway, R. C., Sato, S., Tomomori-Sato, C., Yao, T. & Conaway, J. W. The mammalian Mediator complex and its role in transcriptional regulation. Trends Biochem. Sci. 30, 250–255 (2005).
Kornberg, R. D. Mediator and the mechanism of transcriptional activation. Trends Biochem. Sci. 30, 235–239 (2005).
Lee, T. I. & Young, R. A. Transcription of eukaryotic protein-coding genes. Annu. Rev. Genet. 34, 77–137 (2000).
Boube, M., Joulia, L., Cribbs, D. L. & Bourbon, H. M. Evidence for a mediator of RNA polymerase II transcriptional regulation conserved from yeast to man. Cell 110, 143–151 (2002). One of the first papers to propose and discuss evidence for a mammalian mediator complex.
Sato, S. et al. A set of consensus mammalian mediator subunits identified by multidimensional protein identification technology. Mol. Cell 14, 685–691 (2004).
Bourbon, H. M. Comparative genomics supports a deep evolutionary origin for the large, four-module transcriptional mediator complex. Nucleic Acids Res. 36, 3993–4008 (2008).
Malik, S. & Roeder, R. G. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nature Rev. Genet. 11, 761–772 (2010).
Takahashi, H. et al. Human mediator subunit MED26 functions as a docking site for transcription elongation factors. Cell 146, 92–104 (2011).
Zhou, Q., Li, T. & Price, D. H. RNA Polymerase II Elongation Control. Annu Rev. Biochem. 81, 119–143 (2012).
Conaway, R. C. & Conaway, J. W. The Mediator complex and transcription elongation. Biochim. Biophys. Acta 1829, 69–75 (2013).
Napoli, C., Sessa, M., Infante, T. & Casamassimi, A. Unraveling framework of the ancestral Mediator complex in human diseases. Biochimie 94, 579–587 (2012).
Firestein, R. et al. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature 455, 547–551 (2008). Identification of the gene encoding CDK8 as part of a chromosomal region recurrently amplified in colon cancer and a possible mechanism that could link overexpression of CDK8 with β-catenin hyperactivity.
Firestein, R. & Hahn, W. C. Revving the Throttle on an oncogene: CDK8 takes the driver seat. Cancer Res. 69, 7899–7901 (2009).
Chen, W. & Roeder, R. G. Mediator-dependent nuclear receptor function. Semin. Cell Dev. Biol. 22, 749–758 (2011).
Ito, M. et al. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol. Cell 3, 361–370 (1999).
Gu, W. et al. A novel human SRB/MED-containing cofactor complex, SMCC, involved in transcription regulation. Mol. Cell 3, 97–108 (1999).
Rachez, C. et al. A novel protein complex that interacts with the vitamin D3 receptor in a ligand-dependent manner and enhances VDR transactivation in a cell-free system. Genes Dev. 12, 1787–1800 (1998).
Yuan, C. X., Ito, M., Fondell, J. D., Fu, Z. Y. & Roeder, R. G. The TRAP220 component of a thyroid hormone receptor-associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc. Natl Acad. Sci. USA 95, 7939–7944 (1998).
Kang, Y. K., Guermah, M., Yuan, C. X. & Roeder, R. G. The TRAP/mediator coactivator complex interacts directly with estrogen receptors alpha and beta through the TRAP220 subunit and directly enhances estrogen receptor function in vitro. Proc. Natl Acad. Sci. USA 99, 2642–2647 (2002).
Jia, Y. et al. Transcription coactivator PBP, the peroxisome proliferator-activated receptor (PPAR)-binding protein, is required for PPARalpha-regulated gene expression in liver. J. Biol. Chem. 279, 24427–24434 (2004).
Zhang, X. T. et al. MED1/TRAP220 exists predominantly in a TRAP/mediator subpopulation enriched in RNA polymerase II and is required for ER-mediated transcription. Mol. Cell 19, 89–100 (2005).
Vijayvargia, R., May, M. S. & Fondell, J. D. A coregulatory role for the mediator complex in prostate cancer cell proliferation and gene expression. Cancer Res. 67, 4034–4041 (2007). This paper reports the increased expression of MED1 in prostate cancer and that knockdown of MED1reduces androgen-dependent transcription.
Lamy, P. J. et al. Quantification and clinical relevance of gene amplification at chromosome 17q12-q21 in human epidermal growth factor receptor 2-amplified breast cancers. Breast Cancer Res. 13, R15 (2011).
Nagalingam, A. et al. Med1 plays a critical role in the development of tamoxifen resistance. Carcinogenesis 33, 918–930 (2012).
Cui, J. et al. Cross-talk between HER2 and MED1 regulates tamoxifen resistance of human breast cancer cells. Cancer Res. 72, 5625–5634 (2012).
Kim, H. J. et al. Loss of Med1/TRAP220 promotes the invasion and metastasis of human non-small-cell lung cancer cells by modulating the expression of metastasis-related genes. Cancer Lett. 321, 195–202 (2012).
Barbieri, C. E. et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nature Genet. 44, 685–689 (2012).
Li, L. H., He, J., Hua, D., Guo, Z. J. & Gao, Q. Lentivirus-mediated inhibition of Med19 suppresses growth of breast cancer cells in vitro. Cancer Chemother. Pharmacol. 68, 207–215 (2011).
Sun, M. et al. MED19 promotes proliferation and tumorigenesis of lung cancer. Mol. Cell Biochem. 355, 27–33 (2011).
Zou, S. W. et al. The role of Med19 in the proliferation and tumorigenesis of human hepatocellular carcinoma cells. Acta Pharmacol. Sin. 32, 354–360 (2011).
Zhang, H. et al. Expression of Med19 in bladder cancer tissues and its role on bladder cancer cell growth. Urol. Oncol. 30, 920–927 (2012).
Kuuselo, R. et al. Intersex-like (IXL) is a cell survival regulator in pancreatic cancer with 19q13 amplification. Cancer Res. 67, 1943–1949 (2007).
Chen, S. et al. Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification. Cancer Biol. Ther. 7, 1793–1802 (2008).
Kuuselo, R., Savinainen, K., Sandstrom, S., Autio, R. & Kallioniemi, A. MED29, a component of the mediator complex, possesses both oncogenic and tumor suppressive characteristics in pancreatic cancer. Int. J. Cancer 129, 2553–2565 (2011). This paper reports results demonstrating attenuation of malignant potential in pancreatic cells with MED29 knockdown.
Boyer, T. G., Martin, M. E., Lees, E., Ricciardi, R. P. & Berk, A. J. Mammalian Srb/Mediator complex is targeted by adenovirus E1A protein. Nature 399, 276–279 (1999).
Blattner, C. et al. Molecular basis of Rrn3-regulated RNA polymerase I initiation and cell growth. Genes Dev. 25, 2093–2105 (2011).
Soutourina, J., Wydau, S., Ambroise, Y., Boschiero, C. & Werner, M. Direct interaction of RNA polymerase II and mediator required for transcription in vivo. Science 331, 1451–1454 (2011).
Takagi, Y. et al. Head module control of mediator interactions. Mol. Cell 23, 355–364 (2006).
Zhao, J., Yuan, X., Frodin, M. & Grummt, I. ERK-dependent phosphorylation of the transcription initiation factor TIF-IA is required for RNA polymerase I transcription and cell growth. Mol. Cell 11, 405–413 (2003).
Mayer, C. Zhao, J., Yuan, X. & Grummt, I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 18, 423–434 (2004).
Hoppe, S. et al. AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply. Proc. Natl Acad. Sci. USA 106, 17781–17786 (2009).
Bierhoff, H., Dundr, M., Michels, A. A. & Grummt, I. Phosphorylation by casein kinase 2 facilitates rRNA gene transcription by promoting dissociation of TIF-IA from elongating RNA polymerase I. Mol. Cell. Biol. 28, 4988–4998 (2008).
Johnson, S. S., Zhang, C., Fromm, J., Willis, I. M. & Johnson, D. L. Mammalian Maf1 is a negative regulator of transcription by all three nuclear RNA polymerases. Mol. Cell 26, 367–379 (2007).
Willis, I. M. & Moir, R. D. Integration of nutritional and stress signaling pathways by Maf1. Trends Biochem. Sci. 32, 51–53 (2007). A review of the mechanisms by which the transcriptional co-repressor MAF1 regulates RNA Pol III transcription.
Wei, Y. & Zheng, X. S. Maf1 regulation: a model of signal transduction inside the nucleus. Nucleus 1, 162–165 (2010).
Gilchrist, D. A. et al. Pausing of RNA polymerase II disrupts DNA-specified nucleosome organization to enable precise gene regulation. Cell 143, 540–551 (2010).
Phatnani, H. P. & Greenleaf, A. L. Phosphorylation & functions of the R. N. A. polymerase I. I. C. T. D. Genes Dev. 20, 2922–2936 (2006).
Yamada, T. et al. P-TEFb-mediated phosphorylation of hSpt5 C-terminal repeats is critical for processive transcription elongation. Mol. Cell 21, 227–237 (2006).
Gilmour, D. S. & Lis, J. T. RNA polymerase II interacts with the promoter region of the noninduced hsp70 gene in Drosophila melanogaster cells. Mol. Cell. Biol. 6, 3984–3989 (1986).
Rougvie, A. E. & Lis, J. T. The RNA polymerase II molecule at the 5' end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell 54, 795–804 (1988).
Krumm, A., Meulia, T., Brunvand, M. & Groudine, M. The block to transcriptional elongation within the human c-myc gene is determined in the promoter-proximal region. Genes Dev. 6, 2201–2213 (1992).
Plet, A., Eick, D. & Blanchard, J. M. Elongation and premature termination of transcripts initiated from c-fos and c-myc promoters show dissimilar patterns. Oncogene 10, 319–328 (1995).
Zeitlinger, J. et al. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nature Genet. 39, 1512–1516 (2007).
Muse, G. W. et al. RNA polymerase is poised for activation across the genome. Nature Genet. 39, 1507–1511 (2007). References 110 and 111 present some of the first evidence that RNA Pol transcription is highly regulated at the level of elongation, and that this may be particularly relevant in regulating the expression of genes involved in development.
Gilchrist, D. A. et al. NELF-mediated stalling of Pol II can enhance gene expression by blocking promoter-proximal nucleosome assembly. Genes Dev. 22, 1921–1933 (2008).
Nechaev, S. et al. Global analysis of short RNAs reveals widespread promoter-proximal stalling and arrest of Pol II in Drosophila. Science 327, 335–338 (2010).
Singh, J. & Padgett, R. A. Rates of in situ transcription and splicing in large human genes. Nature Struct. Mol. Biol. 16, 1128–1133 (2009).
Orphanides, G., LeRoy, G., Chang, C. H., Luse, D. S. & Reinberg, D. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell 92, 105–116 (1998).
Belotserkovskaya, R. et al. FACT facilitates transcription-dependent nucleosome alteration. Science 301, 1090–1093 (2003).
Orphanides, G., Wu, W. H., Lane, W. S., Hampsey, M. & Reinberg, D. The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature 400, 284–288 (1999).
Smith, E., Lin, C. & Shilatifard, A. The super elongation complex (SEC) and MLL in development and disease. Genes Dev. 25, 661–672 (2011).
Daser, A. & Rabbitts, T. H. The versatile mixed lineage leukaemia gene MLL and its many associations in leukaemogenesis. Semin. Cancer Biol. 15, 175–188 (2005).
Luo, Z., Lin, C. & Shilatifard, A. The super elongation complex (SEC) family in transcriptional control. Nature Rev. Mol. Cell Biol. 13, 543–547 (2012). Contemporary review highlighting the role of the SEC and MLL in normal RNA Pol transcription elongation, and how this is perturbed in malignancies expressing MLL fusions.
Xu, Q., Nakanishi, T., Sekimizu, K. & Natori, S. Cloning and identification of testis-specific transcription elongation factor S-II. J. Biol. Chem. 269, 3100–3103 (1994).
Labhart, P. & Morgan, G. T. Identification of novel genes encoding transcription elongation factor TFIIS (TCEA) in vertebrates: conservation of three distinct TFIIS isoforms in frog, mouse, and human. Genomics 52, 278–288 (1998).
Shema, E., Kim, J., Roeder, R. G. & Oren, M. RNF20 inhibits TFIIS-facilitated transcriptional elongation to suppress pro-oncogenic gene expression. Mol. Cell 42, 477–488 (2011).
Scotto, L. et al. Identification of copy number gain and overexpressed genes on chromosome arm 20q by an integrative genomic approach in cervical cancer: potential role in progression. Genes Chromosomes Cancer 47, 755–765 (2008).
Hubbard, K., Catalano, J., Puri, R. K. & Gnatt, A. Knockdown of TFIIS by RNA silencing inhibits cancer cell proliferation and induces apoptosis. BMC Cancer 8, 133 (2008).
Yoshida, K. et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 478, 64–69 (2011).
Visconte, V., Makishima, H., Maciejewski, J. P. & Tiu, R. V. Emerging roles of the spliceosomal machinery in myelodysplastic syndromes and other hematological disorders. Leukemia 26, 2447–2454 (2012).
Morris, A. R. et al. Alternative cleavage and polyadenylation during colorectal cancer development. Clin. Cancer Res. 18, 5256–5266 (2012).
Mayr, C. & Bartel, D. P. Widespread shortening of 3'UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 138, 673–684 (2009).
Singh, P. et al. Global changes in processing of mRNA 3' untranslated regions characterize clinically distinct cancer subtypes. Cancer Res. 69, 9422–9430 (2009).
Kaida, D., Schneider-Poetsch, T. & Yoshida, M. Splicing in oncogenesis and tumor suppression. Cancer Sci. 103, 1611–1616 (2012).
Ghavi-Helm, Y. et al. Genome-wide location analysis reveals a role of TFIIS in RNA polymerase III transcription. Genes Dev. 22, 1934–1947 (2008).
Birch, J. L. et al. FACT facilitates chromatin transcription by RNA polymerases I and III. EMBO J. 28, 854–865 (2009).
Stefanovsky, V., Langlois, F., Gagnon-Kugler, T., Rothblum, L. I. & Moss, T. Growth factor signaling regulates elongation of RNA polymerase I transcription in mammals via UBF phosphorylation and r-chromatin remodeling. Mol. Cell 21, 629–639 (2006). This paper presents data that indicate that RNA Pol I transcription can be regulated at the level of elongation by changes in UBF phosphorylation status.
Chan, J. C. et al. AKT promotes rRNA synthesis and cooperates with c-MYC to stimulate ribosome biogenesis in cancer. Sci Signal 4, ra56 (2011).
Robinson, M. J. & Cobb, M. H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9, 180–186 (1997).
Poortinga, G. et al. MAD1 and c-MYC regulate UBF and rDNA transcription during granulocyte differentiation. EMBO J. 23, 3325–3335 (2004). First paper describing MYC regulation of RNA Pol I transcription.
Huang, R. et al. Upstream binding factor up-regulated in hepatocellular carcinoma is related to the survival and cisplatin-sensitivity of cancer cells. FASEB J. 16, 293–301 (2002).
Hannan, K. M. et al. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol. Cell. Biol. 23, 8862–8877 (2003).
Schneider, D. A. RNA polymerase I activity is regulated at multiple steps in the transcription cycle: recent insights into factors that influence transcription elongation. Gene 493, 176–184 (2012).
Anderson, S. J. et al. The transcription elongation factor Spt5 influences transcription by RNA polymerase I positively and negatively. J. Biol. Chem. 286, 18816–18824 (2011).
Viktorovskaya, O. V., Appling, F. D. & Schneider, D. A. Yeast transcription elongation factor Spt5 associates with RNA polymerase I and RNA polymerase II directly. J. Biol. Chem. 286, 18825–18833 (2011).
Zhang, Y., Sikes, M. L., Beyer, A. L. & Schneider, D. A. The Paf1 complex is required for efficient transcription elongation by RNA polymerase I. Proc. Natl Acad. Sci. USA 106, 2153–2158 (2009).
Drygin, D., Rice, W. G. & Grummt, I. The RNA polymerase I transcription machinery: an emerging target for the treatment of cancer. Annu. Rev. Pharmacol. Toxicol. 50, 131–156 (2010).
Darnell, J. E. Jr. Transcription factors as targets for cancer therapy. Nature Rev. Cancer 2, 740–749 (2002). A seminal review of the development of inhibitors of RNA Pol transcription to be used clinically in cancer treatment.
Koehler, A. N. A complex task? Direct modulation of transcription factors with small molecules. Curr. Opin. Chem. Biol. 14, 331–340 (2010).
Morishita, R. et al. In vivo transfection of cis element “decoy” against nuclear factor-kappaB binding site prevents myocardial infarction. Nature Med. 3, 894–899 (1997).
De Stefano, D., De Rosa, G. & Carnuccio, R. NFkappaB decoy oligonucleotides. Curr. Opin. Mol. Ther. 12, 203–213 (2010).
Gambari, R. New trends in the development of transcription factor decoy (TFD) pharmacotherapy. Curr. Drug Targets 5, 419–430 (2004).
De Stefano, D. Oligonucleotides decoy to NF-kappaB: becoming a reality? Discov. Med. 12, 97–105 (2011).
Koh, J. T. & Zheng, J. The new biomimetic chemistry: artificial transcription factors. ACS Chem. Biol. 2, 599–601 (2007).
Jamieson, A. C., Miller, J. C. & Pabo, C. O. Drug discovery with engineered zinc-finger proteins. Nature Rev. Drug Discov. 2, 361–368 (2003).
Bogdanove, A. J. & Voytas, D. F. TAL effectors: customizable proteins for DNA targeting. Science 333, 1843–1846 (2011).
Zhang, F. et al. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nature Biotechnol. 29, 149–153 (2011).
Boch, J. et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326, 1509–1512 (2009).
Krystof, V., Baumli, S. & Furst, R. Perspective of cyclin-dependent kinase 9 (CDK9) as a drug target. Curr. Pharm. Des. 18, 2883–2890 (2012). A contemporary review of the development of inhibitors of CDK9 towards their application in cancer treatment.
Godley, L. A. & Larson, R. A. Therapy-related myeloid leukemia. Semin. Oncol. 35, 418–429 (2008).
Hijiya, N., Ness, K. K., Ribeiro, R. C. & Hudson, M. M. Acute leukemia as a secondary malignancy in children and adolescents: current findings and issues. Cancer 115, 23–35 (2009).
Boutet, S. et al. High-resolution protein structure determination by serial femtosecond crystallography. Science 337, 362–364 (2012).
Zhai, W. & Comai, L. Repression of RNA polymerase I transcription by the tumor suppressor p53. Mol. Cell. Biol. 20, 5930–5938 (2000).
Crighton, D. et al. p53 represses RNA polymerase III transcription by targeting TBP and inhibiting promoter occupancy by TFIIIB. EMBO J. 22, 2810–2820 (2003).
Budde, A. & Grummt, I. p53 represses ribosomal gene transcription. Oncogene 18, 1119–1124 (1999). The first paper to describe the inhibition of RNA Pol I transcription by p53.
Stein, T., Crighton, D., Boyle, J. M., Varley, J. M. & White, R. J. RNA polymerase III transcription can be derepressed by oncogenes or mutations that compromise p53 function in tumours and Li-Fraumeni syndrome. Oncogene 21, 2961–2970 (2002). The initial paper describing the inhibition fo RNA Pol III transcription by p53.
Grewal, S. S., Li, L., Orian, A., Eisenman, R. N. & Edgar, B. A. Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nature Cell Biol. 7, 295–302 (2005).
Arabi, A. et al. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nature Cell Biol. 7, 303–310 (2005).
Grandori, C. et al. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nature Cell Biol. 7, 311–318 (2005). References 165 and 166 were the first papers to describe the direct binding of MYC to the ribosomal gene repeats.
Shiue, C. N., Berkson, R. G. & Wright, A. P. c-Myc induces changes in higher order rDNA structure on stimulation of quiescent cells. Oncogene 28, 1833–1842 (2009).
Kenneth, N. S. et al. TRRAP and GCN5 are used by c-Myc to activate RNA polymerase III transcription. Proc. Natl Acad. Sci. USA 104, 14917–14922 (2007).
Jordan, P. & Carmo-Fonseca, M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell. Mol. Life Sci. 57, 1229–1235 (2000).
Siddik, Z. H. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22, 7265–7279 (2003).
Gniazdowski, M., Denny, W. A., Nelson, S. M. & Czyz, M. Transcription factors as targets for DNA-interacting drugs. Curr. Med. Chem. 10, 909–924 (2003).
Longley, D. B., Harkin, D. P. & Johnston, P. G. 5-fluorouracil: mechanisms of action and clinical strategies. Nature Rev. Cancer 3, 330–338 (2003).
Ghoshal, K. & Jacob, S. T. Specific inhibition of pre-ribosomal RNA processing in extracts from the lymphosarcoma cells treated with 5-fluorouracil. Cancer Res. 54, 632–636 (1994).
Iapalucci-Espinoza, S. & Franze-Fernandez, M. T. Effect of protein synthesis inhibitors and low concentrations of actinomycin D on ribosomal RNA synthesis. FEBS Lett. 107, 281–284 (1979).
Lempiainen, H. & Shore, D. Growth control and ribosome biogenesis. Curr. Opin. Cell Biol. 21, 855–863 (2009).
Granneman, S. & Baserga, S. J. Ribosome biogenesis: of knobs and RNA processing. Exp. Cell Res. 296, 43–50 (2004).
Moss, T., Langlois, F., Gagnon-Kugler, T. & Stefanovsky, V. A housekeeper with power of attorney: the rRNA genes in ribosome biogenesis. Cell. Mol. Life Sci. 64, 29–49 (2007).
Russell, J. & Zomerdijk, J. C. RNA-polymerase-I-directed rDNA transcription, life and works. Trends Biochem. Sci. 30, 87–96 (2005).
Hernandez-Verdun, D. Assembly and disassembly of the nucleolus during the cell cycle. Nucleus 2, 189–194 (2011).
McStay, B. & Grummt, I. The epigenetics of rRNA genes: from molecular to chromosome biology. Annu. Rev. Cell Dev. Biol. 24, 131–157 (2008).
Ruggero, D. & Pandolfi, P. P. Does the ribosome translate cancer? Nature Rev. Cancer 3, 179–192 (2003).
Derenzini, M., Montanaro, L. & Trere, D. What the nucleolus says to a tumour pathologist. Histopathology 54, 753–762 (2009).
Boisvert, F. M., van Koningsbruggen, S., Navascues, J. & Lamond, A. I. The multifunctional nucleolus. Nature Rev. Mol. Cell Biol. 8, 574–585 (2007).
Pederson, T. The nucleolus. Cold Spring Harb. Perspect. Biol. 1 Mar 2011 (doi:10.1101/cshperspect.a000638).
Ruggero, D. Revisiting the nucleolus: from marker to dynamic integrator of cancer signaling. Sci Signal 5, pe38 (2012).
Boulon, S., Westman, B. J., Hutten, S., Boisvert, F. M. & Lamond, A. I. The nucleolus under stress. Mol. Cell 40, 216–227 (2010).
Deisenroth, C. & Zhang, Y. Ribosome biogenesis surveillance: probing the ribosomal protein-Mdm2-p53 pathway. Oncogene 29, 4253–4260 (2010). References 177 and 178 are two excellent reviews on the current understanding of how cells sense and respond to changes in the nucleolus, with particular emphasis on the role of p53 and nucleolar stress.
Fumagalli, S. et al. Absence of nucleolar disruption after impairment of 40S ribosome biogenesis reveals an rpL11-translation-dependent mechanism of p53 induction. Nature Cell Biol. 11, 501–508 (2009).
Russell, J. & Zomerdijk, J. C. The RNA polymerase I transcription machinery. Biochem. Soc. symp. 203–216 (2006).
Roeder, R. G. Lasker Basic Medical Research Award. The eukaryotic transcriptional machinery: complexities and mechanisms unforeseen. Nature Med. 9, 1239–1244 (2003).
Acknowledgements
Owing to scope and space limitations the authors have not been able to individually cite many of the original publications that have contributed substantially to the field. The authors sincerely apologize to the authors of these publications. This work was supported by the National Health and Medical Research Council (NHMRC) of Australia programme and project grants; Leukemia Foundation Grant in Aid; Prostate Cancer Foundation of Australia grant in aid; NHMRC Research Fellowship to R.D.H. and R.B.P.; NHMRC Postgraduate Research Scholarship, GSK Postgraduate Research Scholarship and Leukemia Foundation Postdoctoral Fellowship to M.J.B.; Cancer Council of Victoria Sir Edward Weary Dunlop Clinical Research Fellowship and NHMRC Clinical Research Fellowship to G.A.M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
DATABASES
FURTHER INFORMATION
Glossary
- Mediator complex
-
A multiprotein complex that functions as an RNA Pol II transcriptional co-activator in all eukaryotes, although it is unable to bind specific DNA sequences, it functions as an adaptor between sequence-specific transcription factors bound at regulatory elements, and RNA Pol II and GTFs.
- General transcription factors
-
(GTFs). Also known as basal transcriptional factors. A class of protein transcription factors that bind to specific sites on DNA to activate transcription and are essential for basal (as opposed to activated) RNA Pol I, RNA Pol II and RNA Pol III transcription.
- TATA box
-
A DNA sequence (cis-regulatory element) that is found in the core promoter region of a subset of RNA Pol II and RNA Pol III genes. For RNA Pol II the TATA box is involved in recruiting the RNA Pol II general transcription factor (GTF) transcription initiation factor IID (TFIID) that contains TATA-box-binding protein (TBP).
- SL-1
-
An essential transcription initiation factor complex unique to RNA Pol I consisting of TATA-box-binding protein (TBP) and at least five TBP-associated factors (TAFs) that functions to recruit RNA Pol I to the ribosomal RNA gene promoters.
- Nucleotide excision repair
-
A DNA repair mechanism that removes mutations resulting from ultraviolet-induced DNA damage.
- Xeroderma pigmentosum
-
An autosomal recessive genetic disorder of nucleotide excision repair caused by mutations in at least eight separate genes including XPC, ERCC2 and POLH, in which the ability to repair DNA damage caused by ultraviolet light is deficient.
- Pre-initiation complex
-
(PIC). The complex of proteins that is necessary for the initiation of RNA Pol I, RNA Pol II and RNA Pol III transcription in eukaryotes where it helps position RNA Pol over gene transcription start sites, denatures the DNA, and positions the DNA in the RNA Pol active site for transcription.
- Capping
-
The process in which a guanine nucleotide is connected to the 5′ end of newly RNA Pol II transcribed mRNA via an unusual 5′ to 5′ triphosphate linkage that facilitates nuclear export of the mRNA, prevents its degradation by exonucleases, and promotes translation and 5′ proximal intron excision.
- Pausing
-
A control step in gene transcription by which RNA Pol II pauses ∼20–40 nucleotides downstream of the transcription start site and requires specific stimuli and elongation factors to overcome the pausing block to enter productive elongation.
- C-terminal domain
-
(CTD). The extended CTD of RPB1 that is phosphorylated at Ser2 and Ser5 in the 52 heptad repeat sequence, YSPTSPS, which is crucial for promoter escape and elongation.
- Super elongation complex
-
(SEC). Consists of the RNA Pol II elongation factors including ELL proteins, positive transcription elongation factor (P-TEFb) and several mixed lineage leukaemia (MLL) translocation partners and is required for rapid transcriptional induction in the presence or absence of paused RNA Pol II.
- Homeobox (HOX) genes
-
A highly conserved family of homeodomain-containing transcription factors involved in the control of early development, including haematopoiesis, the dysregulation of which is associated with a number of haematological malignancies.
- DNA-alkylating agents
-
The oldest class of anticancer drugs still commonly used for cancer treatment that function as methylating agents that form adducts at the O and N atoms in DNA.
- Nucleotide analogues
-
Among the first chemotherapeutic agents to be introduced for the medical treatment of cancer, they include a variety of purine and pyrimidine nucleotide derivatives that function as antimetabolites and compete with physiological nucleotides to induce cytotoxicity.
- Anthracyclines
-
A class of drugs used in cancer chemotherapy derived from Streptomyces bacterium Streptomyces peucetius var. caesius, which function by intercalating between base pairs of the DNA–RNA strand, thus preventing DNA and RNA synthesis; they also inhibit topoisomerase II, which leads to DNA breaks.
- DNA crosslinks
-
DNA crosslinks (either intrastrand or interstrand) block DNA replication and/or DNA transcription and occur when various exogenous or endogenous agents react with two different positions in the DNA to form covalent adducts with DNA bases.
- Platinum compounds
-
A class of platinum-containing anticancer drugs (for example, cisplatin) that function by crosslinking DNA.
- Peptide nucleic acids
-
(PNAs). Nucleic acid analogues in which the sugar–phosphate backbone of natural nucleic acids has been replaced by a synthetic peptide backbone, resulting in an achiral and uncharged mimic that is capable of sequence-specific recognition of DNA and RNA.
- Transcription activator-like effectors
-
(TALEs). A class of naturally occurring DNA-binding proteins found in the plant pathogen Xanthomonas spp. in which the DNA-binding domain of each TALE consists of tandem 34-amino acid repeat modules that can be rearranged according to a simple cipher to target new DNA sequences for the use in a wide variety of genome-engineering applications, including transcriptional modulation.
- Micro-focus beam lines
-
To obtain structures from increasingly small crystals (<20 micrometers), synchrotron facilities have developed micro-focus beam lines or high-intensity beams with a small focal spot. The demand to use even smaller crystals, (<10 micrometers), has necessitated the development of a 'mini-beam' apparatus.
- Third-generation synchrotron radiation sources
-
Third-generation radiation sources, either low energy (1–2 GeV) or high energy (6–8 GeV), use novel insertion devices in synchrotron storage rings to enhance the intensity of the radiation source, thus providing two or more orders of magnitude higher brightness than earlier generation sources.
- Free electron lasers
-
Produce ultra-intense, ultra-short X-ray pulses that have applications in exploring new frontiers in science research such as studying atoms in motion, chemical reactions and phase transitions in materials with atomic resolution on the femtosecond timescale.
Rights and permissions
About this article
Cite this article
Bywater, M., Pearson, R., McArthur, G. et al. Dysregulation of the basal RNA polymerase transcription apparatus in cancer. Nat Rev Cancer 13, 299–314 (2013). https://doi.org/10.1038/nrc3496
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3496
This article is cited by
-
From signalling pathways to targeted therapies: unravelling glioblastoma’s secrets and harnessing two decades of progress
Signal Transduction and Targeted Therapy (2023)
-
Dynamic de novo heterochromatin assembly and disassembly at replication forks ensures fork stability
Nature Cell Biology (2023)
-
Cullin-5 neddylation-mediated NOXA degradation is enhanced by PRDX1 oligomers in colorectal cancer
Cell Death & Disease (2021)
-
ERK signalling: a master regulator of cell behaviour, life and fate
Nature Reviews Molecular Cell Biology (2020)
-
BRD4 prevents the accumulation of R-loops and protects against transcription–replication collision events and DNA damage
Nature Communications (2020)