Key Points
-
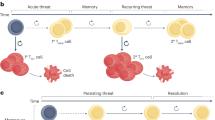
T lymphocytes transition through progressive stages of differentiation that are characterized by a stepwise loss of functional and therapeutic potential.
-
Subsets of mature T cells exhibit the stem cell-like attributes of self-renewal, multipotency and the ability to undergo asymmetric division.
-
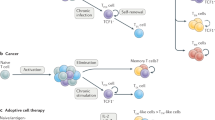
Evolutionarily conserved pathways regulating stemness are active in T cells, including T memory stem cells, T helper 17 cells and interleukin-17 (IL-17)-producing CD8+ T cells.
-
Pharmacological and genetic induction of stem cell pathways can be used to generate tumour-specific T cells with stem cell-like properties.
-
Reprogramming terminally differentiated tumour-reactive T cells to display naive or stem cell-like functionalities might be obtained through the expression of transcription factors or microRNAs that are associated with naive or T memory stem cells.
-
Stem cell-like T cells possess enhanced capacities to engraft, persist and mediate prolonged immune attack against tumour masses that are sustained by long-lived cancer stem cells.
Abstract
Stem cells are defined by the ability to self-renew and to generate differentiated progeny, qualities that are maintained by evolutionarily conserved pathways that can lead to cancer when deregulated. There is now evidence that these stem cell-like attributes and signalling pathways are also shared among subsets of mature memory T lymphocytes. We discuss how using stem cell-like T cells can overcome the limitations of current adoptive T cell therapies, including inefficient T cell engraftment, persistence and ability to mediate prolonged immune attack. Conferring stemness to antitumour T cells might unleash the full potential of cellular therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Weissman, I. L. Stem cells: units of development, units of regeneration, and units in evolution. Cell 100, 157–168 (2000).
Spangrude, G. J., Heimfeld, S. & Weissman, I. L. Purification and characterization of mouse hematopoietic stem cells. Science 241, 58–62 (1988).
Reynolds, B. A. & Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707–1710 (1992).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Sun, T. T. & Green, H. Differentiation of the epidermal keratinocyte in cell culture: formation of the cornified envelope. Cell 9, 511–521 (1976).
Beltrami, A. P. et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 (2003).
Rawlins, E. L. et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4, 525–534 (2009).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Nguyen, L. V., Vanner, R., Dirks, P. & Eaves, C. J. Cancer stem cells: an evolving concept. Nature Rev. Cancer 12, 133–143 (2012).
Kaech, S. M., Wherry, E. J. & Ahmed, R. Effector and memory T-cell differentiation: implications for vaccine development. Nature Rev. Immunol. 2, 251–262 (2002).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 (2004).
Klebanoff, C. A., Gattinoni, L. & Restifo, N. P. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol. Rev. 211, 214–224 (2006).
Gett, A. V., Sallusto, F., Lanzavecchia, A. & Geginat, J. T cell fitness determined by signal strength. Nature Immunol. 4, 355–360 (2003).
D'Souza, W. N. & Hedrick, S. M. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J. Immunol. 177, 777–781 (2006).
Sarkar, S. et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 205, 625–640 (2008).
Chattopadhyay, P. K. et al. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nature Med. 12, 972–977 (2006).
Newell, E. W., Sigal, N., Bendall, S. C., Nolan, G. P. & Davis, M. M. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity 36, 142–152 (2012).
Ahmed, R., Bevan, M. J., Reiner, S. L. & Fearon, D. T. The precursors of memory: models and controversies. Nature Rev. Immunol. 9, 662–668 (2009).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nature Med. 17, 1290–1297 (2011). This paper identifies a new subset of memory T cells with enhanced stem cell-like properties in humans and shows that these cells mediate a superior antitumour response in a mouse model of adoptive T cell therapy.
De Rosa, S. C., Herzenberg, L. A., Herzenberg, L. A. & Roederer, M. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nature Med. 7, 245–248 (2001).
Weninger, W., Crowley, M. A., Manjunath, N. & von Andrian, U. H. Migratory properties of naive, effector, and memory CD8+ T cells. J. Exp. Med. 194, 953–966 (2001).
Oberdoerffer, S. et al. Regulation of CD45 alternative splicing by heterogeneous ribonucleoprotein, hnRNPLL. Science 321, 686–691 (2008).
Wu, Z. et al. Memory T cell RNA rearrangement programmed by heterogeneous nuclear ribonucleoprotein hnRNPLL. Immunity 29, 863–875 (2008).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Henson, S. M. et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood 113, 6619–6628 (2009).
Romero, P. et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J. Immunol. 178, 4112–4119 (2007).
Kambayashi, T., Assarsson, E., Lukacher, A. E., Ljunggren, H. G. & Jensen, P. E. Memory CD8+ T cells provide an early source of IFN-γ. J. Immunol. 170, 2399–2408 (2003).
Judge, A. D., Zhang, X., Fujii, H., Surh, C. D. & Sprent, J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J. Exp. Med. 196, 935–946 (2002).
Tan, J. T. et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 195, 1523–1532 (2002).
Hamann, D. et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186, 1407–1418 (1997).
Siegel, R. M., Chan, F. K., Chun, H. J. & Lenardo, M. J. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nature Immunol. 1, 469–474 (2000).
Stadler, B. M. & Ruohola-Baker, H. Small RNAs: keeping stem cells in line. Cell 132, 563–566 (2008).
Ma, F. et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nature Immunol. 12, 861–869 (2011).
Steiner, D. F. et al. MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells. Immunity 35, 169–181 (2011).
O'Connell, R. M. et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 33, 607–619 (2010).
Wu, H. et al. miRNA profiling of naive, effector and memory CD8 T cells. PLoS ONE. 2, e1020 (2007).
Salaun, B. et al. Differentiation associated regulation of microRNA expression in vivo in human CD8+ T cell subsets. J. Transl. Med. 9, 44 (2011).
Ranganathan, P. et al. Regulation of acute graft-versus-host disease by microRNA-155. Blood 119, 4786–4797 (2012).
Fearon, D. T., Manders, P. & Wagner, S. D. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science 293, 248–250 (2001). This perspective article is the first to draw a parallel between stem cells and memory B and T lymphocytes.
Stemberger, C. et al. Stem cell-like plasticity of naive and distinct memory CD8+ T cell subsets. Semin. Immunol. 21, 62–68 (2009).
Luckey, C. J. et al. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc. Natl Acad. Sci. USA 103, 3304–3309 (2006). This paper describes a common transcriptional signature shared between memory T cells, memory B cells and long-term haematopoietic stem cells, providing systems biology evidence for Fearon's hypothesis.
Knoblich, J. A. Mechanisms of asymmetric stem cell division. Cell 132, 583–597 (2008).
Chang, J. T. et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315, 1687–1691 (2007). This paper provides the first evidence that asymmetric cell division can occur in mature T cells to confer memory and effector cell fates to daughter cells.
Barnett, B. E. et al. Asymmetric B cell division in the germinal center reaction. Science 335, 342–344 (2012).
Ciocca, M. L., Barnett, B. E., Burkhardt, J. K., Chang, J. T. & Reiner, S. L. Cutting edge: asymmetric memory T cell division in response to rechallenge. J. Immunol. 188, 4145–4148 (2012).
Kalia, V. et al. Prolonged interleukin-2Rα expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32, 91–103 (2010).
Joshi, N. S. et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-BET transcription factor. Immunity 27, 281–295 (2007).
Morrison, S. J., Prowse, K. R., Ho, P. & Weissman, I. L. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity 5, 207–216 (1996).
Hodes, R. J., Hathcock, K. S. & Weng, N. P. Telomeres in T and B cells. Nature Rev. Immunol. 2, 699–706 (2002).
Sallusto, F. & Lanzavecchia, A. Memory in disguise. Nature Med. 17, 1182–1183 (2011).
Zhang, Y., Joe, G., Hexner, E., Zhu, J. & Emerson, S. G. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nature Med. 11, 1299–1305 (2005). This paper describes a memory stem cell-like CD8+ T cell population in a mouse model of graft-versus-host disease.
Gattinoni, L. et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nature Med. 15, 808–813 (2009).
Lugli, E., Gattinoni, L., Restifo, N. P. & Roederer, M. The role of T memory stem cells: pathogenesis and vaccines. J. Acquir. Immune Defic. Syndr. 59, 59 (2012).
Papagno, L. et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS. Biol. 2, e20 (2004).
Chang, H. H., Hemberg, M., Barahona, M., Ingber, D. E. & Huang, S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature 453, 544–547 (2008).
Morrison, S. J. & Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008).
Eckfeldt, C. E., Mendenhall, E. M. & Verfaillie, C. M. The molecular repertoire of the 'almighty' stem cell. Nature Rev. Mol. Cell. Biol. 6, 726–737 (2005).
Williams, R. L. et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 336, 684–687 (1988).
Niwa, H., Burdon, T., Chambers, I. & Smith, A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 12, 2048–2060 (1998).
Matsuda, T. et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 18, 4261–4269 (1999).
Hall, J. et al. Oct4 and LIF/Stat3 additively induce Kruppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell 5, 597–609 (2009).
Burdon, T., Stracey, C., Chambers, I., Nichols, J. & Smith, A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev. Biol. 210, 30–43 (1999).
Qu, C. K. & Feng, G. S. Shp-2 has a positive regulatory role in ES cell differentiation and proliferation. Oncogene 17, 433–439 (1998).
Li, Z. et al. BMP4 Signaling Acts via dual-specificity phosphatase 9 to control ERK activity in mouse embryonic stem cells. Cell Stem Cell 10, 171–182 (2012).
Ying, Q. L., Nichols, J., Chambers, I. & Smith, A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115, 281–292 (2003).
Alarcon, C. et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell 139, 757–769 (2009).
Lian, I. et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 24, 1106–1118 (2010).
Tamm, C., Bower, N. & Anneren, C. Regulation of mouse embryonic stem cell self-renewal by a Yes-YAP-TEAD2 signaling pathway downstream of LIF. J. Cell Sci. 124, 1136–1144 (2011).
Leonard, W. J. & Spolski, R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nature Rev. Immunol. 5, 688–698 (2005).
Hinrichs, C. S. et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 111, 5326–5333 (2008).
Yi, J. S., Du, M. & Zajac, A. J. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324, 1572–1576 (2009).
Frohlich, A. et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324, 1576–1580 (2009).
Yi, J. S., Ingram, J. T. & Zajac, A. J. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J. Immunol. 185, 4835–4845 (2010).
Cui, W., Liu, Y., Weinstein, J. S., Craft, J. & Kaech, S. M. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35, 792–805 (2011).
Wirth, T. C. et al. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8+ T cell differentiation. Immunity 33, 128–140 (2010).
Betz, U. A. & Muller, W. Regulated expression of gp130 and IL-6 receptor α chain in T cell maturation and activation. Int. Immunol. 10, 1175–1184 (1998).
Castellino, F. & Germain, R. N. Chemokine-guided CD4+ T cell help enhances generation of IL-6RαhighIL-7Rα high prememory CD8+ T cells. J. Immunol. 178, 778–787 (2007).
Siegel, A. M. et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity 35, 806–818 (2011). Together with reference 74, this paper identifies STAT3 as a key transcription factor required for the formation and maintenance of T cell memory in mice and humans.
Yamada, T., Park, C. S., Mamonkin, M. & Lacorazza, H. D. Transcription factor ELF4 controls the proliferation and homing of CD8+ T cells via the Kruppel-like factors KLF4 and KLF2. Nature Immunol. 10, 618–626 (2009).
Weinreich, M. A. et al. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity 31, 122–130 (2009).
Ji, Y. et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nature Immunol. 12, 1230–1237 (2011).
Yang, C. Y. et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nature Immunol. 12, 1221–1229 (2011). References 81 and 82 show that the DNA-binding inhibitor ID3 is crucial for the formation of long-lived memory.
D'Cruz, L. M., Lind, K. C., Wu, B. B., Fujimoto, J. K. & Goldrath, A. W. Loss of E protein transcription factors E2A and HEB delays memory-precursor formation during the CD8+ T-cell immune response. Eur. J Immunol. 42, 2031–2041 (2012).
Thaventhiran, J. E. et al. Activation of the Hippo pathway by CTLA-4 regulates the expression of Blimp-1 in the CD8+ T cell. Proc. Natl Acad. Sci. USA 27 Jun 2012 (doi:10.1073/pnas.1209115109).
Muranski, P. et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 35, 972–985 (2011).
Luckey, C. J. & Weaver, C. T. Stem-cell-like qualities of immune memory; CD4+ T cells join the party. Cell Stem Cell 10, 107–108 (2012).
Kryczek, I. et al. Human TH17 cells are long-lived effector memory cells. Sci. Transl. Med. 3, 104ra100 (2011). This paper, along with reference 85, provides evidence that T H 17 cells are a long-lived memory T cell population that retain stem cell-like attributes in mice and humans.
Wray, J. & Hartmann, C. WNTing embryonic stem cells. Trends Cell Biol. 22, 159–168 (2012).
ten, B. D. et al. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nature Cell Biol. 13, 1070–1075 (2011).
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P. & Brivanlou, A. H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Med. 10, 55–63 (2004).
Kielman, M. F. et al. Apc modulates embryonic stem-cell differentiation by controlling the dosage of β-catenin signaling. Nature Genet. 32, 594–605 (2002).
Doble, B. W., Patel, S., Wood, G. A., Kockeritz, L. K. & Woodgett, J. R. Functional redundancy of GSK-3α and GSK-3β in Wnt/β-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev. Cell 12, 957–971 (2007).
Wray, J. et al. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nature Cell Biol. 13, 838–845 (2011).
Yi, F. et al. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nature Cell Biol. 13, 762–770 (2011).
Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 (2006).
Jeannet, G. et al. Long-term, multilineage hematopoiesis occurs in the combined absence of β-catenin and γ-catenin. Blood 111, 142–149 (2008).
Willinger, T. et al. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J. Immunol. 176, 1439–1446 (2006).
Gattinoni, L., Ji, Y. & Restifo, N. P. Wnt/β-catenin signaling in T-cell immunity and cancer immunotherapy. Clin. Cancer Res. 16, 4695–4701 (2010).
Xue, H. H. & Zhao, D. M. Regulation of mature T cell responses by the Wnt signaling pathway. Ann. NY Acad. Sci. 1247, 16–33 (2012).
Gattinoni, L. Ji, Y. & Restifo, N. P. β-catenin does not regulate memory T cell phenotype Reply. Nature Med. 16, 514–515 (2010).
Muralidharan, S. et al. Activation of Wnt signaling arrests effector differentiation in human peripheral and cord blood-derived T lymphocytes. J. Immunol. 187, 5221–5232 (2011).
Driessens, G. et al. β-catenin inhibits T cell activation by selective interference with linker for activation of T cells-phospholipase C-γ1 phosphorylation. J. Immunol. 186, 784–790 (2011).
Zhao, D. M. et al. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J. Immunol. 184, 1191–1199 (2010).
Jeannet, G. et al. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc. Natl Acad. Sci. USA 107, 9777–9782 (2010).
Zhou, X. et al. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity 33, 229–240 (2010). References 104 and 105 show that TCF1–β-catenin signalling is essential for the establishment and maintenance of functional CD8+ T cell memory.
Driessens, G., Zheng, Y. & Gajewski, T. F. β-catenin does not regulate memory T cell phenotype. Nature Med. 16, 513–514 (2010).
Prlic, M. & Bevan, M. J. Cutting edge: β-catenin is dispensable for T cell effector differentiation, memory formation, and recall responses. J. Immunol. 187, 1542–1546 (2011).
Huelsken, J. & Held, W. Canonical Wnt signalling plays essential roles. Eur. J. Immunol. 39, 3582–3583 (2009).
Staal, F. J. & Sen, J. M. Canonical Wnt signalling plays essential roles. Author reply. Eur. J. Immunol. 39, 3583–3584 (2009).
Maeda, O. et al. Plakoglobin (γ-catenin) has TCF/LEF family-dependent transcriptional activity in β-catenin-deficient cell line. Oncogene 23, 964–972 (2004).
Murakami, M. et al. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol. Cell. Biol. 24, 6710–6718 (2004).
Sampath, P. et al. A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell 2, 448–460 (2008).
Easley, C. A. et al. mTOR-mediated activation of p70 S6K induces differentiation of pluripotent human embryonic stem cells. Cell Reprogram. 12, 263–273 (2010).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Yang, K., Neale, G., Green, D. R., He, W. & Chi, H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nature Immunol. 12, 888–897 (2011).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009). Together with reference 114, this paper provides evidence that the commitment of a T cell between effector or memory fates is regulated by key, pharmacologically targetable, metabolic pathways.
Kim, E. H. et al. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J. Immunol. 188, 4305–4314 (2012).
Macintyre, A. N. et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity 34, 224–236 (2011).
Hand, T. W. et al. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc. Natl Acad. Sci. USA 107, 16601–16606 (2010).
Rao, R. R., Li, Q., Odunsi, K. & Shrikant, P. A. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-BET and Eomesodermin. Immunity 32, 67–78 (2010).
Rao, R. R., Li, Q., Gubbels Bupp, M. R. & Shrikant, P. A. Transcription factor foxo1 represses t-bet-mediated effector functions and promotes memory CD8+ T cell differentiation. Immunity 36, 374–387 (2012).
Sinclair, L. V. et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nature Immunol. 9, 513–521 (2008).
Kerdiles, Y. M. et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nature Immunol. 10, 176–184 (2009).
Tang, T. T. et al. The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J. Biol. Chem. 277, 14255–14265 (2002).
Zhang, X. et al. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nature Cell Biol. 13, 1092–1099 (2011).
Kolb, H. J. et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 86, 2041–2050 (1995).
Kochenderfer, J. N. et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012).
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 (2011).
Bollard, C. M. et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood 110, 2838–2845 (2007).
Rosenberg, S. A. et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17, 4550–4557 (2011).
Robbins, P. F. et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924 (2011).
Klebanoff, C. A., Khong, H. T., Antony, P. A., Palmer, D. C. & Restifo, N. P. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 26, 111–117 (2005).
Robbins, P. F. et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J. Immunol. 173, 7125–7130 (2004).
Pule, M. A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature Med. 14, 1264–1270 (2008).
Zhou, J. et al. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J. Immunol. 175, 7046–7052 (2005).
Schwartzentruber, D. J. et al. In vitro predictors of therapeutic response in melanoma patients receiving tumor-infiltrating lymphocytes and interleukin-2. J. Clin. Oncol. 12, 1475–1483 (1994).
Besser, M. J. et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 16, 2646–2655 (2010).
Louis, C. U. et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011).
Huang, J. et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J. Immunol. 176, 7726–7735 (2006).
Berger, C. et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 118, 294–305 (2008).
Wang, X. et al. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood 117, 1888–1898 (2011).
Sussman, J. J., Parihar, R., Winstead, K. & Finkelman, F. D. Prolonged culture of vaccine-primed lymphocytes results in decreased antitumor killing and change in cytokine secretion. Cancer Res. 64, 9124–9130 (2004).
Gattinoni, L. et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 115, 1616–1626 (2005). This paper is the first to conceptualize and provide experimental evidence demonstrating the detrimental effect of T cell differentiation on the antitumour activity of CD8+ T lymphocytes used for adoptive T cell-based therapy.
Klebanoff, C. A. et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl Acad. Sci. USA 102, 9571–9576 (2005).
Le, H. K. et al. Incubation of antigen-sensitized T lymphocytes activated with bryostatin 1 + ionomycin in IL-7 + IL-15 increases yield of cells capable of inducing regression of melanoma metastases compared to culture in IL-2. Cancer Immunol. Immunother. 58, 1565–1576 (2009).
Stark, F. C., Sad, S. & Krishnan, L. Intracellular bacterial vectors that induce CD8(+) T cells with similar cytolytic abilities but disparate memory phenotypes provide contrasting tumor protection. Cancer Res. 69, 4327–4334 (2009).
Klebanoff, C. A. et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin. Cancer Res. 17, 5343–5352 (2011).
Topalian, S. L., Muul, L. M., Solomon, D. & Rosenberg, S. A. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J. Immunol. Methods 102, 127–141 (1987).
Riddell, S. R. et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 257, 238–241 (1992).
Gattinoni, L., Powell, D. J. Jr, Rosenberg, S. A. & Restifo, N. P. Adoptive immunotherapy for cancer: building on success. Nature Rev. Immunol. 6, 383–393 (2006).
Gattinoni, L., Klebanoff, C. A. & Restifo, N. P. Pharmacologic induction of CD8+ T cell memory: better living through chemistry. Sci. Transl. Med. 1, 11ps12 (2009).
Manjunath, N. et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Invest. 108, 871–878 (2001).
van der Windt, G. J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
Butler, M. O. et al. Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells. Sci. Transl. Med. 3, 80ra34 (2011).
Zeng, R. et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 201, 139–148 (2005).
Li, Y., Bleakley, M. & Yee, C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J. Immunol. 175, 2261–2269 (2005).
Albrecht, J. et al. IL-21-treated naive CD45RA+ CD8+ T cells represent a reliable source for producing leukemia-reactive cytotoxic T lymphocytes with high proliferative potential and early differentiation phenotype. Cancer Immunol. Immunother. 60, 235–248 (2011).
Pearce, E. L. Metabolism in T cell activation and differentiation. Curr. Opin. Immunol. 22, 314–320 (2010).
Aberle, H. Bauer, A., Stappert, J., Kispert, A. & Kemler, R. β-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16, 3797–3804 (1997).
Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nature Rev. Immunol. 12, 325–338 (2012).
Cohen, P. & Goedert, M. GSK3 inhibitors: development and therapeutic potential. Nature Rev. Drug Discov. 3, 479–487 (2004).
Zippelius, A. et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 64, 2865–2873 (2004).
Ahmadzadeh, M. et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544 (2009).
Baitsch, L. et al. Exhaustion of tumor-specific CD8 T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360 (2011).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007). References 165 and 166 provide evidence in mice and humans that a pluripotent stem cell can be artificially derived from an adult somatic cell by forced expression of a limited number of key transcription factors.
Anokye-Danso, F. et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell 8, 376–388 (2011). This paper shows that a single microRNA cluster (microRNA 302–367) can efficiently reprogramme adult somatic cells to acquire pluripotency.
Eminli, S. et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nature Genet. 41, 968–976 (2009).
Loh, Y. H. et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell 7, 15–19 (2010).
Staerk, J. et al. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell 7, 20–24 (2010).
Seki, T. et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell 7, 11–14 (2010).
Schmitt, T. M. et al. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nature Immunol. 5, 410–417 (2004).
Schmitt, T. M. & Zuniga-Pflucker, J. C. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17, 749–756 (2002). This paper describes a simple in vitro culture system that allows for the generation of mature T cells from haematopoietic progenitors.
Clark, R. A., Yamanaka, K., Bai, M., Dowgiert, R. & Kupper, T. S. Human skin cells support thymus-independent T cell development. J. Clin. Invest. 115, 3239–3249 (2005).
Zhao, Y. et al. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling. Cancer Res. 67, 2425–2429 (2007).
Lei, F., Haque, R., Weiler, L., Vrana, K. E. & Song, J. T lineage differentiation from induced pluripotent stem cells. Cell. Immunol. 260, 1–5 (2009).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Marro, S. et al. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell 9, 374–382 (2011).
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010).
Szabo, E. et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 468, 521–526 (2010).
Sekiya, S. & Suzuki, A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 475, 390–393 (2011).
Miura, K. et al. Variation in the safety of induced pluripotent stem cell lines. Nature Biotechnol. 27, 743–745 (2009).
Bonini, C. et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 276, 1719–1724 (1997).
Di, S. A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 365, 1673–1683 (2011).
Wu, S. M. & Hochedlinger, K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nature Cell Biol. 13, 497–505 (2011).
Zhao, T., Zhang, Z. N., Rong, Z. & Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 474, 212–215 (2011).
Wherry, E. J. et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nature Immunol. 4, 225–234 (2003).
Chang, J. T. et al. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity 34, 492–504 (2011).
Appay, V. et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nature Med. 8, 379–385 (2002).
Willinger, T., Freeman, T., Hasegawa, H., McMichael, A. J. & Callan, M. F. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J. Immunol. 175, 5895–5903 (2005).
Holmes, S., He, M., Xu, T. & Lee, P. P. Memory T cells have gene expression patterns intermediate between naive and effector. Proc. Natl Acad. Sci. USA 102, 5519–5523 (2005).
Waddington, C. H. The Strategy of the Genes (George Allen & Unwin, 1957).
Chapuis, A. G. et al. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc. Natl Acad. Sci. USA 109, 4592–4597 (2012).
Swain, S. L., McKinstry, K. K. & Strutt, T. M. Expanding roles for CD4+ T cells in immunity to viruses. Nature Rev. Immunol. 12, 136–148 (2012).
Pepper, M. et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nature Immunol. 11, 83–89 (2010).
Luthje, K. et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nature Immunol. 13, 491–498 (2012).
Nurieva, R. I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008).
Acknowledgements
This work was supported by the National Institutes of Health, Center for Regenerative Medicine (NIH-CRM) and by the Center for Cancer Research (CCR) by the US National Cancer Institute (Bethesda, Maryland). The authors would like to thank M. Rao for helpful discussions about regenerative medicine. M. Bachinski copyedited the manuscript during construction. J. Crompton, R. Roychoudhuri, P. Muranski, D. Palmer, Y. Ji, M. Sukumar, J. Pan, A. Leonardi, Z. Franco, Z. Yu and D. Clever provided a lively sounding board for concepts presented here. J. C. Yang, U. S. Kammula, R. A. Morgan, S. A. Feldman, P. F. Robbins, R. M. Sherry, M. Parkhurst, M. Hughes and G. Phan made many valuable suggestions regarding the clinical translation of the work described. The authors would especially like to thank our longtime stalwart ally in all of these efforts S. A. Rosenberg.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Self-renewal
-
A biological process by which a cell gives rise to one or two daughter cells that have a developmental potential that is indistinguishable from that of the mother cell.
- Multipotency
-
The potential for a cell to give rise to progeny with the capacity to form multiple, but not all possible, lineages.
- Tumour-infiltrating lymphocytes
-
(TILs). The heterogeneous population of T cells found in a tumour bed. These cells are characterized by a diversity of phenotypes, antigen specificities, avidities and functional characteristics. They can be activated and expanded ex vivo and re-infused into a tumour-bearing host to mediate tumour regression.
- Mass cytometry
-
Also known as cytometry by time-of-flight (CyTOF). A platform that couples flow cytometry with mass spectrometry. This technique enables the simultaneous evaluation of at least 45 simultaneous phenotypic and functional parameters on a single cell without the use of fluorescent agents or interference from spectral overlap.
- Senescence
-
A biological process by which cells undergo growth arrest after extensive replication.
- Homeostatic proliferation
-
A process of activation and proliferation of leukocytes in a lymphopaenic environment. T cell homeostatic proliferation is driven by T cell receptor interactions with self-peptide–MHC complexes and responsiveness to homeostatic cytokines such as interleukin-7 (IL-7), IL-15 and possibly IL-21.
- TCR excision circles
-
(TRECs). Circular, stable extra-chromosomal DNA fragments that are generated during recombination of variable (V), diversity (D) and joining regions (J) of the T cell receptor. TRECs do not replicate with cellular proliferation and are thus diluted with every cell division, allowing the assessment of the replicative history of a T cell.
- Asymmetric cell division
-
A conserved mechanism by which a cell divides into daughter cells of unequal size and cytoplasmic content, thus conferring differential developmental fates to progeny cells.
- Telomere
-
The segment at the end of chromosomal arms consisting of a series of repeated DNA sequences (TTAGGG in all vertebrates) that regulates chromosomal replication at each cell division.
- Stem cell niche
-
A specialized microenvironment containing stem cells that supports their maintenance and regulates their function.
- Common γ-chain
-
(γC). A signalling subunit common to the receptors for interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15 and IL-21.
- Exhaustion
-
A state of T cell dysfunction arising during reiterative antigen stimulations such as chronic infections and cancer. It is defined by poor effector function and proliferative response to antigenic stimuli, expression of inhibitory receptors and a transcriptional state that is distinct from that of functional effector or memory T cells.
- Autosomal-dominant hyper-IgE syndrome
-
(AD-HIES). Also known as Job's syndrome. A rare primary immunodeficiency characterized by recurrent skin abscesses, cyst-forming pneumonias and extreme increases of serum IgE levels. Most AD-HIES cases are caused by dominant-negative mutations in STAT3.
- Epigenetic modifications
-
Heritable molecular alterations of the genome that do not involve changes to the nucleotide sequence that regulates gene or microRNA expression. They include DNA methylation, histone modifications and nucleosome positioning.
- Induced pluripotent stem (iPS) cells
-
Pluripotent stem cells artificially derived from non-pluripotent cells, such as an adult somatic cell by forced expression of specific genes or microRNAs.
- Suicide genes
-
Genes capable of selectively eliminating the cells into which they have been transduced following the administration of a drug.
Rights and permissions
About this article
Cite this article
Gattinoni, L., Klebanoff, C. & Restifo, N. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer 12, 671–684 (2012). https://doi.org/10.1038/nrc3322
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3322
This article is cited by
-
Stem-cell-like CD4+ T cells prey on MHC class II–negative tumors
Nature Immunology (2023)
-
RUNX3 improves CAR-T cell phenotype and reduces cytokine release while maintaining CAR-T function
Medical Oncology (2023)
-
Homeostatic cytokines tune naivety and stemness of cord blood-derived transgenic T cells
Cancer Gene Therapy (2022)
-
Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells
Nature Biotechnology (2022)
-
cBAF complex components and MYC cooperate early in CD8+ T cell fate
Nature (2022)