Key Points
-
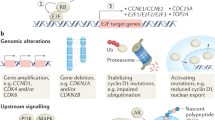
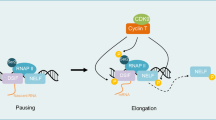
Cyclin D–cyclin-dependent kinase 4 (CDK4) or CDK6 activation promotes cell cycle progression through the phosphorylation of substrates, including RB and transcription factors with roles in proliferation and differentiation. These kinase complexes also target substrates with roles in centrosome duplication, mitochondrial function, cell growth, cell adhesion and motility, and cytoskeletal modelling.
-
D-type cyclins have non-catalytic roles in which interactions with chromatin-modifying enzymes and diverse transcription factors, including steroid hormone receptors, leads to the transcriptional regulation of suites of genes that are involved in proliferation and differentiation. Independently of CDK activation, the D-type cyclins also facilitate efficient DNA repair and indirectly activate CDK2 through the sequestration of CDK inhibitors.
-
CCND1 is an established human oncogene that is commonly overexpressed through copy number alterations, or more rarely by mutation, or as a consequence of the deregulation of mitogenic signalling downstream of oncogenes such as ERBB2. CCND1 overexpression causes a number of potentially oncogenic responses in experimental models and is associated with poor patient outcome.
-
Cyclin D1 and its associated CDKs are potential therapeutic targets. Promising results from early CDK inhibitors in experimental systems were not followed by evidence for efficacy in clinical trials. Possible reasons for this disappointing outcome include poor pharmacokinetics, suboptimal dosing schedules and clinical testing in unselected patient populations. Second-generation, more selective inhibitors of CDK4 and CDK6 are now undergoing clinical testing.
-
Possible alternative approaches to targeting cyclin D1 include the use of compounds that affect CCND1 transcription or cyclin D1 protein turnover, and the use of combination therapies that simultaneously target multiple end points of cyclin D1 action. Central to the effective use of these novel approaches is the better selection of patient subgroups that are likely to respond.
Abstract
Cyclin D1, and to a lesser extent the other D-type cyclins, is frequently deregulated in cancer and is a biomarker of cancer phenotype and disease progression. The ability of these cyclins to activate the cyclin-dependent kinases (CDKs) CDK4 and CDK6 is the most extensively documented mechanism for their oncogenic actions and provides an attractive therapeutic target. Is this an effective means of targeting the cyclin D oncogenes, and how might the patient subgroups that are most likely to benefit be identified?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sherr, C. J. & Roberts, J. M. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18, 2699–2711 (2004).
Besson, A., Dowdy, S. F. & Roberts, J. M. CDK inhibitors: cell cycle regulators and beyond. Dev. Cell 14, 159–169 (2008).
Matsushime, H. et al. Identification and properties of an atypical catalytic subunit (p34PSK–J3/cdk4) for mammalian D type G1 cyclins. Cell 71, 323–334 (1992).
Meyerson, M. & Harlow, E. Identification of a G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell. Biol. 14, 2077–2086 (1994).
Musgrove, E. A. Cyclins: roles in mitogenic signaling and oncogenic transformation. Growth Factors 24, 13–19 (2006).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Malumbres, M. & Barbacid, M. Cell cycle, CDKs and cancer: a changing paradigm. Nature Rev. Cancer 9, 153–166 (2009).
Gil, J. & Peters, G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nature Rev. Mol. Cell. Biol. 7, 667–677 (2006).
Chen, H. Z., Tsai, S. Y. & Leone, G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nature Rev. Cancer 9, 785–797 (2009).
Lukas, J. et al. DNA tumor virus oncoproteins and retinoblastoma gene mutations share the ability to relieve the cell's requirement for cyclin D1 function in G1. J. Cell Biol. 125, 625–638 (1994).
Matsuura, I. et al. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature 430, 226–231 (2004).
Shen, R. et al. Cyclin D1-cdk4 induce runx2 ubiquitination and degradation. J. Biol. Chem. 281, 16347–16353 (2006).
Zhang, L., Fried, F. B., Guo, H. & Friedman, A. D. Cyclin-dependent kinase phosphorylation of RUNX1/AML1 on 3 sites increases transactivation potency and stimulates cell proliferation. Blood 111, 1193–1200 (2008).
Nakajima, K. et al. Coordinated regulation of differentiation and proliferation of embryonic cardiomyocytes by a jumonji (Jarid2)-cyclin D1 pathway. Development 138, 1771–1782 (2011).
Lazaro, J. B., Bailey, P. J. & Lassar, A. B. Cyclin D-cdk4 activity modulates the subnuclear localization and interaction of MEF2 with SRC-family coactivators during skeletal muscle differentiation. Genes Dev. 16, 1792–1805 (2002).
Kehn, K. et al. Functional consequences of cyclin D1/BRCA1 interaction in breast cancer cells. Oncogene 26, 5060–5069 (2007).
Adon, A. M. et al. Cdk2 and Cdk4 regulate the centrosome cycle and are critical mediators of centrosome amplification in p53-null cells. Mol. Cell. Biol. 30, 694–710 (2010).
Wang, C. et al. Cyclin D1 repression of nuclear respiratory factor 1 integrates nuclear DNA synthesis and mitochondrial function. Proc. Natl Acad. Sci. USA 103, 11567–11572 (2006).
Zacharek, S. J., Xiong, Y. & Shumway, S. D. Negative regulation of TSC1-TSC2 by mammalian D-type cyclins. Cancer Res. 65, 11354–11360 (2005).
Aggarwal, P. et al. Nuclear cyclin D1/CDK4 kinase regulates CUL4 expression and triggers neoplastic growth via activation of the PRMT5 methyltransferase. Cancer Cell 18, 329–340 (2010).
Ren, J. et al. Methylation of ribosomal protein S10 by protein-arginine methyltransferase 5 regulates ribosome biogenesis. J. Biol. Chem. 285, 12695–12705 (2010).
Sarcevic, B., Lilischkis, R. & Sutherland, R. L. Differential phosphorylation of T-47D human breast cancer cell substrates by D1-, D3-, E-, and A-cyclin-CDK complexes. J. Biol. Chem. 272, 33327–33337 (1997).
Baker, G. L., Landis, M. W. & Hinds, P. W. Multiple functions of D-type cyclins can antagonize pRb-mediated suppression of proliferation. Cell Cycle 4, 330–338 (2005).
Zhong, Z. et al. Cyclin D1/cyclin-dependent kinase 4 interacts with filamin A and affects the migration and invasion potential of breast cancer cells. Cancer Res. 70, 2105–2114 (2010).
Neumeister, P. et al. Cyclin D1 governs adhesion and motility of macrophages. Mol. Biol. Cell 14, 2005–2015 (2003). This publication showed that cyclin D1 was not solely a regulator of cell proliferation, but also had effects on cellular migration.
Li, Z. et al. Cyclin D1 regulates cellular migration through the inhibition of thrombospondin 1 and ROCK signaling. Mol. Cell. Biol. 26, 4240–4256 (2006).
Li, Z. et al. Cyclin D1 induction of cellular migration requires p27(KIP1). Cancer Res. 66, 9986–9994 (2006).
Chow, Y. H. et al. Role of Cdk4 in lymphocyte function and allergen response. Cell Cycle 9, 4922–4930 (2010).
Bienvenu, F. et al. Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature 463, 374–378 (2010). This publication conclusively shows cyclin D1 binding to DNA during normal mouse development, underlining the importance of its effects as a transcriptional regulator.
McMahon, C., Suthiphongchai, T., DiRenzo, J. & Ewen, M. E. P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc. Natl Acad. Sci. USA 96, 5382–5387 (1999).
Reutens, A. T. et al. Cyclin D1 binds the androgen receptor and regulates hormone-dependent signaling in a p300/CBP-associated factor (P/CAF)-dependent manner. Mol. Endocrinol. 15, 797–811 (2001).
Fu, M. et al. Cyclin D1 inhibits peroxisome proliferator-activated receptor γ-mediated adipogenesis through histone deacetylase recruitment. J. Biol. Chem. 280, 16934–16941 (2005).
Coqueret, O. Linking cyclins to transcriptional control. Gene 299, 35–55 (2002).
Fu, M., Wang, C., Li, Z., Sakamaki, T. & Pestell, R. G. Minireview: cyclin D1: normal and abnormal functions. Endocrinology 145, 5439–5447 (2004).
Mullany, L. K. et al. Distinct proliferative and transcriptional effects of the D-type cyclins in vivo. Cell Cycle 7, 2215–2224 (2008).
Neuman, E. et al. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol. Cell. Biol. 17, 5338–5347 (1997).
Zwijsen, R. M. L. et al. CDK-independent activation of estrogen receptor by cyclin D1. Cell 88, 405–415 (1997).
Despouy, G. et al. Cyclin D3 is a cofactor of retinoic acid receptors, modulating their activity in the presence of cellular retinoic acid-binding protein II. J. Biol. Chem. 278, 6355–6362 (2003).
Sarruf, D. A. et al. Cyclin D3 promotes adipogenesis through activation of peroxisome proliferator-activated receptor γ. Mol. Cell. Biol. 25, 9985–9995 (2005).
Knudsen, K. E., Cavenee, W. K. & Arden, K. C. D-type cyclins complex with the androgen receptor and inhibit its transcriptional transactivation ability. Cancer Res. 59, 2297–2301 (1999). References 36, 37 and 40 were the first to document kinase-independent functions of cyclin D1 in both promoting and inhibiting steroid hormone receptor transcriptional activity.
Zong, H. et al. Cyclin D3/CDK11p58 complex is involved in the repression of androgen receptor. Mol. Cell. Biol. 27, 7125–7142 (2007).
Sherr, C. J. & Roberts, J. M. CDK inhibitors: positive and negative regulators of G1 phase progression. Genes. Dev. 13, 1501–1512 (1999).
Besson, A., Assoian, R. K. & Roberts, J. M. Regulation of the cytoskeleton: an oncogenic function for CDK inhibitors? Nature Rev. Cancer 4, 948–955 (2004).
Jirawatnotai, S. et al. A function for cyclin D1 in DNA repair uncovered by protein interactome analyses in human cancers. Nature 474, 230–234 (2011).
Li, Z. et al. Alternative cyclin D1 splice forms differentially regulate the DNA damage response. Cancer Res. 70, 8802–8811 (2010). References 44 and 45 show that cyclin D1 promotes efficient DNA repair, independently of CDK4/CDK6 activity, through binding to RAD51 and BRCA2.
Raderschall, E. et al. Formation of higher-order nuclear Rad51 structures is functionally linked to p21 expression and protection from DNA damage-induced apoptosis. J. Cell Sci. 115, 153–164 (2002).
Kozar, K. et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell 118, 477–491 (2004).
Malumbres, M. et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 118, 493–504 (2004).
Landis, M. W., Pawlyk, B. S., Li, T., Sicinski, P. & Hinds, P. W. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell 9, 13–22 (2006).
Geng, Y. et al. Deletion of the p27Kip1 gene restores normal development in cyclin D1-deficient mice. Proc. Natl Acad. Sci. USA 98, 194–199 (2001).
Tsutsui, T. et al. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27Kip1 activity. Mol. Cell. Biol. 19, 7011–7019 (1999).
Yasui, M. et al. Antisense to cyclin D1 inhibits vascular endothelial growth factor-stimulated growth of vascular endothelial cells: implication of tumor vascularization. Clin. Cancer Res. 12, 4720–4729 (2006).
Nelsen, C. J. et al. Short term cyclin D1 overexpression induces centrosome amplification, mitotic spindle abnormalities, and aneuploidy. J. Biol. Chem. 280, 768–776 (2005).
Pontano, L. L. et al. Genotoxic stress-induced cyclin D1 phosphorylation and proteolysis are required for genomic stability. Mol. Cell. Biol. 28, 7245–7258 (2008).
Shields, B. J., Hauser, C., Bukczynska, P. E., Court, N. W. & Tiganis, T. DNA replication stalling attenuates tyrosine kinase signaling to suppress S phase progression. Cancer Cell 14, 166–179 (2008).
Aggarwal, P. et al. Nuclear accumulation of cyclin D1 during S phase inhibits Cul4-dependent Cdt1 proteolysis and triggers p53-dependent DNA rereplication. Genes Dev. 21, 2908–2922 (2007).
Yu, Q., Geng, Y. & Sicinski, P. Specific protection against breast cancers by cyclin D1 ablation. Nature 411, 1017–1021 (2001). This paper shows that cyclin D1 is essential for some, but not all, oncogenic pathways, and was followed by a series of publications that together showed that ERRB2.driven mammary oncogenesis required the ability of cyclin D1 to activate CDK4.
Yu, Q. et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 9, 23–32 (2006).
Yang, C. et al. The role of the cyclin D1-dependent kinases in ErbB2-mediated breast cancer. Am. J. Pathol. 164, 1031–1038 (2004).
Hu, M. G. et al. A requirement for cyclin-dependent kinase 6 in thymocyte development and tumorigenesis. Cancer Res. 69, 810–818 (2009).
Sicinska, E. et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 4, 451–461 (2003).
Berthet, C. & Kaldis, P. Cell-specific responses to loss of cyclin-dependent kinases. Oncogene 26, 4469–4477 (2007).
Cole, A. M. et al. Cyclin D2-cyclin-dependent kinase 4/6 is required for efficient proliferation and tumorigenesis following Apc loss. Cancer Res. 70, 8149–8158 (2010).
Kim, J. K. & Diehl, J. A. Nuclear cyclin D1: an oncogenic driver in human cancer. J. Cell. Physiol. 220, 292–296 (2009).
Gautschi, O., Ratschiller, D., Gugger, M., Betticher, D. C. & Heighway, J. Cyclin D1 in non-small cell lung cancer: a key driver of malignant transformation. Lung Cancer 55, 1–14 (2007).
Comstock, C. E., Revelo, M. P., Buncher, C. R. & Knudsen, K. E. Impact of differential cyclin D1 expression and localisation in prostate cancer. Br. J. Cancer 96, 970–979 (2007).
Dhar, K. K. et al. Expression and subcellular localization of cyclin D1 protein in epithelial ovarian tumour cells. Br. J. Cancer 81, 1174–1181 (1999).
Ahmed, K. M., Fan, M., Nantajit, D., Cao, N. & Li, J. J. Cyclin D1 in low-dose radiation-induced adaptive resistance. Oncogene 27, 6738–6748 (2008).
Knudsen, K. E., Diehl, J. A., Haiman, C. A. & Knudsen, E. S. Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene 25, 1620–1628 (2006).
Kim, C. J. et al. Cyclin D1b variant promotes cell invasiveness independent of binding to CDK4 in human bladder cancer cells. Mol. Carcinog. 48, 953–964 (2009).
Li, Z. et al. Alternate cyclin D1 mRNA splicing modulates p27KIP1 binding and cell migration. J. Biol. Chem. 283, 7007–7015 (2008).
Zhu, J., Sen, S., Wei, C. & Frazier, M. L. Cyclin D1b represses breast cancer cell growth by antagonizing the action of cyclin D1a on estrogen receptor α-mediated transcription. Int. J. Oncol. 36, 39–48 (2010).
Burd, C. J. et al. Cyclin D1b variant influences prostate cancer growth through aberrant androgen receptor regulation. Proc. Natl Acad. Sci. USA 103, 2190–2195 (2006).
Santarius, T., Shipley, J., Brewer, D., Stratton, M. R. & Cooper, C. S. A census of amplified and overexpressed human cancer genes. Nature Rev. Cancer 10, 59–64 (2010).
Wang, T. C. et al. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369, 669–671 (1994). This paper shows that cyclin D1 overexpression in the mammary gland is sufficient for tumour formation, the first experimental evidence for its oncogenic capacity in vivo.
Bertoni, F., Rinaldi, A., Zucca, E. & Cavalli, F. Update on the molecular biology of mantle cell lymphoma. Hematol. Oncol. 24, 22–27 (2006).
The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068 (2008).
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010).
Matsubayashi, H. et al. Methylation of cyclin D2 is observed frequently in pancreatic cancer but is also an age-related phenomenon in gastrointestinal tissues. Clin. Cancer Res. 9, 1446–1452 (2003).
Evron, E. et al. Loss of cyclin D2 expression in the majority of breast cancers is associated with promoter hypermethylation. Cancer Res. 61, 2782–2787 (2001).
Padar, A. et al. Inactivation of cyclin D2 gene in prostate cancers by aberrant promoter methylation. Clin. Cancer Res. 9, 4730–4734 (2003).
Wiestner, A. et al. Point mutations and genomic deletions in CCND1 create stable truncated cyclin D1 mRNAs that are associated with increased proliferation rate and shorter survival. Blood 109, 4599–4606 (2007).
Benzeno, S. et al. Identification of mutations that disrupt phosphorylation-dependent nuclear export of cyclin D1. Oncogene 25, 6291–6303 (2006).
Moreno-Bueno, G. et al. Cyclin D1 gene (CCND1) mutations in endometrial cancer. Oncogene 22, 6115–6118 (2003).
Barbash, O. et al. Mutations in Fbx4 inhibit dimerization of the SCF(Fbx4) ligase and contribute to cyclin D1 overexpression in human cancer. Cancer Cell 14, 68–78 (2008).
Russell, A. et al. Cyclin D1 and D3 associate with the SCF complex and are coordinately elevated in breast cancer. Oncogene 18, 1983–1991 (1999).
Pabalan, N. et al. Cyclin D1 Pro241Pro (CCND1-G870A) polymorphism is associated with increased cancer risk in human populations: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 17, 2773–2781 (2008).
Li, R. et al. Expression of cyclin D1 splice variants is differentially associated with outcome in non-small cell lung cancer patients. Hum. Pathol. 39, 1792–1801 (2008).
Millar, E. K. et al. Cyclin D1b protein expression in breast cancer is independent of cyclin D1a and associated with poor disease outcome. Oncogene 28, 1812–1820 (2009).
Comstock, C. E. et al. Cyclin D1 splice variants: polymorphism, risk, and isoform-specific regulation in prostate cancer. Clin. Cancer Res. 15, 5338–5349 (2009).
Abramson, V. G. et al. Cyclin D1b in human breast carcinoma and coexpression with cyclin D1a is associated with poor outcome. Anticancer Res. 30, 1279–1285 (2010).
Sanchez, G., Delattre, O., Auboeuf, D. & Dutertre, M. Coupled alteration of transcription and splicing by a single oncogene: boosting the effect on cyclin D1 activity. Cell Cycle 7, 2299–2305 (2008).
Zeng, X. et al. The Ras oncogene signals centrosome amplification in mammary epithelial cells through cyclin D1/Cdk4 and Nek2. Oncogene 29, 5103–5112 (2010).
Lee, R. J. et al. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol. Cell. Biol. 20, 672–83 (2000).
Desai, K. V. et al. Initiating oncogenic event determines gene-expression patterns of human breast cancer models. Proc. Natl Acad. Sci. USA 99, 6967–6972 (2002).
Ahnstrom, M., Nordenskjold, B., Rutqvist, L. E., Skoog, L. & Stal, O. Role of cyclin D1 in ErbB2-positive breast cancer and tamoxifen resistance. Breast Cancer Res. Treat. 91, 145–151 (2005).
Reis-Filho, J. S. et al. Cyclin D1 protein overexpression and CCND1 amplification in breast carcinomas: an immunohistochemical and chromogenic in situ hybridisation analysis. Mod. Pathol. 19, 999–1009 (2006).
Bandi, N. et al. miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res. 69, 5553–5559 (2009).
Bonci, D. et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nature Med. 14, 1271–1277 (2008).
Jiang, Q., Feng, M. G. & Mo, Y. Y. Systematic validation of predicted microRNAs for cyclin D1. BMC Cancer 9, 194 (2009).
Ewen, M. E. & Lamb, J. The activities of cyclin D1 that drive tumorigenesis. Trends Mol. Med. 10, 158–162 (2004).
Arnold, A. & Papanikolaou, A. Cyclin D1 in breast cancer pathogenesis. J. Clin. Oncol. 23, 4215–4224 (2005).
Rosenwald, A. et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell 3, 185–197 (2003). This analysis found a correlation between cyclin D1 expression and a proliferation gene expression signature.
Jares, P., Colomer, D. & Campo, E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nature Rev. Cancer 7, 750–762 (2007).
Bova, R. J. et al. Cyclin D1 and p16INK14A expression predict reduced survival in carcinoma of the anterior tongue. Clin. Cancer Res. 5, 2810–2819 (1999).
Lamb, J. et al. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell 114, 323–334 (2003). In contrast to reference 103, by demonstrating that cyclin D1 expression was not correlated with an E2F1 and E2F2.activated gene signature, this paper called into question the idea that the proliferative effects of cyclin D1 were responsible for its oncogenic action.
Ertel, A. et al. RB-pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle 9, 4153–4163 (2010).
Agarwal, R. et al. Integrative analysis of cyclin protein levels identifies cyclin b1 as a classifier and predictor of outcomes in breast cancer. Clin. Cancer Res. 15, 3654–3662 (2009).
Sakamaki, T. et al. Cyclin D1 determines mitochondrial function in vivo. Mol. Cell. Biol. 26, 5449–5469 (2006).
Yang, C. et al. Identification of cyclin D1- and estrogen-regulated genes contributing to breast carcinogenesis and progression. Cancer Res. 66, 11649–11658 (2006).
Sweeney, K. J., Swarbrick, A., Sutherland, R. L. & Musgrove, E. A. Lack of relationship between CDK activity and G1 cyclin expression in breast cancer cells. Oncogene 16, 2865–2878 (1998).
Thomas, G. R., Nadiminti, H. & Regalado, J. Molecular predictors of clinical outcome in patients with head and neck squamous cell carcinoma. Int. J. Exp. Pathol. 86, 347–363 (2005).
Roy, P. G. & Thompson, A. M. Cyclin D1 and breast cancer. Breast 15, 718–727 (2006).
Taneja, P. et al. Classical and novel prognostic markers for breast cancer and their clinical significance. Clin. Med. Insights Oncol. 4, 15–34 (2010).
Lukas, J., Bartkova, J., Rodhe, M., Strauss, M. & Bartek, J. Cyclin D1 is dispensible for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol. Cell. Biol. 15, 2600–2611 (1995).
Knudsen, E. S. & Knudsen, K. E. Tailoring to RB: tumour suppressor status and therapeutic response. Nature Rev. Cancer 8, 714–724 (2008).
Kenny, F. S. et al. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin. Cancer Res. 5, 2069–2076 (1999).
Elsheikh, S. et al. CCND1 amplification and cyclin D1 expression in breast cancer and their relation with proteomic subgroups and patient outcome. Breast Cancer Res. Treat. 109, 325–335 (2008).
Biliran, H. Jr. et al. Overexpression of cyclin D1 promotes tumor cell growth and confers resistance to cisplatin-mediated apoptosis in an elastase-myc transgene-expressing pancreatic tumor cell line. Clin. Cancer Res. 11, 6075–6086 (2005).
Kornmann, M. et al. Inhibition of cyclin D1 expression in human pancreatic cancer cells is associated with increased chemosensitivity and decreased expression of multiple chemoresistance genes. Cancer Res. 59, 3505–3511 (1999).
Musgrove, E. A. & Sutherland, R. L. Biological determinants of endocrine resistance in breast cancer. Nature Rev. Cancer 9, 631–643 (2009).
Kalish, L. H. et al. Deregulated cyclin D1 expression is associated with decreased efficacy of the selective epidermal growth factor receptor tyrosine kinase inhibitor gefitinib in head and neck squamous cell carcinoma cell lines. Clin. Cancer Res. 10, 7764–7774 (2004).
Smalley, K. S. et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol. Cancer Ther. 7, 2876–2883 (2008).
Noel, E. E. et al. The association of CCND1 overexpression and cisplatin resistance in testicular germ cell tumors and other cancers. Am. J. Pathol. 176, 2607–2615 (2010).
Shimura, T. et al. Acquired radioresistance of human tumor cells by DNA-PK/AKT/GSK3β-mediated cyclin D1 overexpression. Oncogene 29, 4826–4837 (2010).
Rudas, M. et al. Cyclin D1 expression in breast cancer patients receiving adjuvant tamoxifen-based therapy. Clin. Cancer Res. 14, 1767–1774 (2008).
Stendahl, M. et al. Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br. J. Cancer 90, 1942–1948 (2004).
Weinstein, I. B. & Joe, A. K. Mechanisms of disease: oncogene addiction-a rationale for molecular targeting in cancer therapy. Nature Clin. Pract. Oncol. 3, 448–457 (2006).
Krause, D. S. & Van Etten, R. A. Tyrosine kinases as targets for cancer therapy. N. Engl. J. Med. 353, 172–187 (2005).
Lapenna, S. & Giordano, A. Cell cycle kinases as therapeutic targets for cancer. Nature Rev. Drug Discov. 8, 547–566 (2009).
Shapiro, G. I. Cyclin-dependent kinase pathways as targets for cancer treatment. J. Clin. Oncol. 24, 1770–1783 (2006).
Jeselsohn, R. et al. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell 17, 65–76 (2010).
Fry, D. W. et al. Cell cycle and biochemical effects of PD 0183812. A potent inhibitor of the cyclin D-dependent kinases Cdk4 and Cdk6. J. Biol. Chem. 276, 16617–16623 (2001). This publication shows that a specific inhibitor of CDK4 and CDK6 has anti-proliferative effects, and was followed by a series of publications demonstrating antitumour effects in various malignancies.
Fry, D. W. et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 3, 1427–1438 (2004).
Marzec, M. et al. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood 108, 1744–1750 (2006).
Finn, R. S. et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 11, R77 (2009). In this study a panel of breast cancer cell lines was used to identify a gene expression signature correlated with response to CDK4/CDK6 inhibition. It was the first to use a large-scale, unbiased approach that aimed to develop criteria for patient selection in clinical studies of CDK4/CDK6 inhibition.
Michaud, K. et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 70, 3228–3238 (2010).
Wiedemeyer, W. R. et al. Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc. Natl Acad. Sci. USA 107, 11501–11506 (2010).
Konecny, G. E. et al. Expression of p16 and Retinoblastoma determines response to CDK 4/6 inhibition in ovarian cancer. Clin. Cancer Res. 17, 1591–1602 (2011).
Tan, A. R. et al. Phase I trial of the cyclin-dependent kinase inhibitor flavopiridol in combination with docetaxel in patients with metastatic breast cancer. Clin. Cancer Res. 10, 5038–5047 (2004).
Haddad, R. I. et al. A phase II clinical and pharmacodynamic study of E7070 in patients with metastatic, recurrent, or refractory squamous cell carcinoma of the head and neck: modulation of retinoblastoma protein phosphorylation by a novel chloroindolyl sulfonamide cell cycle inhibitor. Clin. Cancer Res. 10, 4680–4687 (2004).
Berkofsky-Fessler, W. et al. Preclinical biomarkers for a cyclin-dependent kinase inhibitor translate to candidate pharmacodynamic biomarkers in phase I patients. Mol. Cancer Ther. 8, 2517–2525 (2009).
Locatelli, G. et al. Transcriptional analysis of an E2F gene signature as a biomarker of activity of the cyclin-dependent kinase inhibitor PHA-793887 in tumor and skin biopsies from a phase I clinical study. Mol. Cancer Ther. 9, 1265–1273 (2010).
Diab, S. et al. A phase I study of R547, a novel, selective inhibitor of cell cycle and transcriptional cyclin dependent kinases (CDKs). ASCO Meeting Abstr. 25, 3528 (2007).
Schwartz, G. K. et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1). Br. J. Cancer 104, 1862–1868 (2011).
O'Dwyer, P. J. et al. A phase I dose escalation trial of a daily oral CDK 4/6 inhibitor PD-0332991. ASCO Meeting Abstr. 25, 3550 (2007).
Dean, J. L., Thangavel, C., McClendon, A. K., Reed, C. A. & Knudsen, E. S. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene 29, 4018–4032 (2010).
Wang, L. et al. Pharmacologic inhibition of CDK4/6: mechanistic evidence for selective activity or acquired resistance in acute myeloid leukemia. Blood 110, 2075–2083 (2007).
Hanse, E. A. et al. Cdk2 plays a critical role in hepatocyte cell cycle progression and survival in the setting of cyclin D1 expression in vivo. Cell Cycle 8, 2802–2809 (2009).
Bagella, L. et al. A small molecule based on the pRb2/p130 spacer domain leads to inhibition of cdk2 activity, cell cycle arrest and tumor growth reduction in vivo. Oncogene 26, 1829–1839 (2007).
Arris, C. E. et al. Identification of novel purine and pyrimidine cyclin-dependent kinase inhibitors with distinct molecular interactions and tumor cell growth inhibition profiles. J. Med. Chem. 43, 2797–2804 (2000).
Gondeau, C. et al. Design of a novel class of peptide inhibitors of cyclin-dependent kinase/cyclin activation. J. Biol. Chem. 280, 13793–13800 (2005).
Canela, N. et al. Identification of an hexapeptide that binds to a surface pocket in cyclin A and inhibits the catalytic activity of the complex cyclin-dependent kinase 2-cyclin A. J. Biol. Chem. 281, 35942–35953 (2006).
Adams, P. D. et al. Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol. Cell. Biol. 16, 6623–6633 (1996).
Ball, K. L., Lain, S., Fahraeus, R., Smythe, C. & Lane, D. P. Cell-cycle arrest and inhibition of Cdk4 activity by small peptides based on the carboxy-terminal domain of p21WAF1. Curr. Biol. 7, 71–80 (1997).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Day, P. J. et al. Crystal structure of human CDK4 in complex with a D-type cyclin. Proc. Natl Acad. Sci. USA 106, 4166–4170 (2009).
Takaki, T. et al. The structure of CDK4/cyclin D3 has implications for models of CDK activation. Proc. Natl Acad. Sci. USA 106, 4171–4176 (2009).
Schiewer, M. J. et al. Cyclin D1 repressor domain mediates proliferation and survival in prostate cancer. Oncogene 28, 1016–1027 (2009).
Wang, M. et al. Cyclin D1 as a universally expressed mantle cell lymphoma-associated tumor antigen for immunotherapy. Leukemia 23, 1320–1328 (2009).
Dragnev, K. H. et al. Bexarotene and erlotinib for aerodigestive tract cancer. J. Clin. Oncol. 23, 8757–8764 (2005).
Kim, E. S., Lee, J. J. & Wistuba, II. Cotargeting cyclin D1 starts a new chapter in lung cancer prevention and therapy. Cancer Prev. Res. 4, 779–782 (2011).
Kim, E. S. et al. The BATTLE trial: personalising therapy for lung cancer. Cancer Discovery 1, 44–53 (2011).
Dragnev, K. H. et al. Bexarotene plus erlotinib suppress lung carcinogenesis independent of KRAS mutations in two clinical trials and transgenic models. Cancer Prev. Res. 4, 818–828 (2011).
Sabatini, D. M. mTOR and cancer: insights into a complex relationship. Nature Rev. Cancer 6, 729–734 (2006).
Alao, J. P. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol. Cancer 6, 24 (2007).
Shan, J., Zhao, W. & Gu, W. Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol. Cell 36, 469–476 (2009). This study identifies USP2 as a specific deubiquitylase for cyclin D1, and suggests that targeting it may be an effective therapy in cyclin D1.dependent cancers.
Kelland, L. R. in Inhibitors of Cyclin-Dependent Kinases as Anti-Tumor Agents (eds Smith, P. J. & Yue, E. W.) 371–388 (CRC Press, 2007).
Menu, E. et al. A novel therapeutic combination using PD 0332991 and bortezomib: study in the 5T33MM myeloma model. Cancer Res. 68, 5519–5523 (2008).
Baughn, L. B. et al. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 66, 7661–7667 (2006).
Kuo, T. C., Chavarria-Smith, J. E., Huang, D. & Schlissel, M. S. Forced expression of CDK6 confers resistance of pro-B ALL to Gleevec treatment. Mol. Cell. Biol. 31, 2566–2576 (2011).
Johnson, S. M. et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J. Clin. Invest. 120, 2528–2536 (2010).
Biankin, A. V. & Hudson, T. J. Somatic variation and cancer: therapies lost in the mix. Hum. Genet. 5 Jun 2011 (doi:10.1007/s00439-011-1010-0).
Puyol, M. et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 18, 63–73 (2010). This study identifies an interaction between CDKs and RAS signalling that could be used as a basis for the rational design of combination therapies. The synthetic-lethal approach adopted in this study merits wider application, given the tissue specificity of dependence on individual D.type cyclins, CDK4 and CDK6 that is apparent in knockout mice.
Roberts, P. J., Stinchcombe, T. E., Der, C. J. & Socinski, M. A. Personalized medicine in non-small-cell lung cancer: is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J. Clin. Oncol. 28, 4769–4777 (2010).
Zwijsen, R. M., Buckle, R. S., Hijmans, E. M., Loomans, C. J. & Bernards, R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 12, 3488–3498 (1998).
Siegert, J. L., Rushton, J. J., Sellers, W. R., Kaelin, W. G. Jr. & Robbins, P. D. Cyclin D1 suppresses retinoblastoma protein-mediated inhibition of TAFII250 kinase activity. Oncogene 19, 5703–5711 (2000).
Hardisson, D. Molecular pathogenesis of head and neck squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 260, 502–508 (2003).
Moreno-Bueno, G. et al. Molecular alterations associated with cyclin D1 overexpression in endometrial cancer. Int. J. Cancer 110, 194–200 (2004).
Wu, W. et al. Correlation of cyclin D1 and cyclin D3 overexpression with the loss of PTEN expression in endometrial carcinoma. Int. J. Gynecol. Cancer 16, 1668–1672 (2006).
Li, W. et al. The role of cell cycle regulatory proteins in the pathogenesis of melanoma. Pathology 38, 287–301 (2006).
Garcea, G., Neal, C. P., Pattenden, C. J., Steward, W. P. & Berry, D. P. Molecular prognostic markers in pancreatic cancer: a systematic review. Eur. J. Cancer 41, 2213–2236 (2005).
Toncheva, D. et al. Tissue microarray analysis of cyclin D1 gene amplification and gain in colorectal carcinomas. Tumour Biol. 25, 157–160 (2004).
McKay, J. A. et al. Cyclin D1 protein expression and gene polymorphism in colorectal cancer. Aberdeen Colorectal Initiative. Int. J. Cancer 88, 77–81 (2000).
Bergsagel, P. L. & Kuehl, W. M. Molecular pathogenesis and a consequent classification of multiple myeloma. J. Clin. Oncol. 23, 6333–6338 (2005).
Siemeister, G. et al. Pharmacologic profile of the oral novel pan-CDK inhibitor BAY 1000394 in chemosensitive and chemorefractory tumor models. Cancer Res. Abstr. 70, 3883 (2010).
DePinto, W. et al. In vitro and in vivo activity of R547: a potent and selective cyclin-dependent kinase inhibitor currently in phase I clinical trials. Mol. Cancer Ther. 5, 2644–2658 (2006).
Cirstea, D. et al. RGB 286638, a Novel Multi-Targeted Small Molecule Inhibitor, Induces Multiple Myeloma (MM) Cell Death through Abrogation of CDKDependent and Independent Survival Mechanisms. ASH Annual Meeting Abstr. 112, 2759 (2008).
Siemeister, G. et al. Molecular and pharmacodynamic characteristics of the novel multi-target tumor growth inhibitor ZK 304709. Biomed. Pharmacother. 60, 269–272 (2006).
Scott, E. N. et al. A phase I dose escalation study of the pharmacokinetics and tolerability of ZK 304709, an oral multi-targeted growth inhibitor (MTGI), in patients with advanced solid tumours. Cancer Chemother. Pharmacol. 64, 425–429 (2009).
Acknowledgements
The authors are grateful to C. M. McNeil and C. M. Sergio for assistance with literature searches, A. V. Biankin for thought-provoking discussions and I. Rooman for helpful comments. The authors' research is supported by the National Health and Medical Research Council of Australia, Cancer Institute New South Wales, National Breast Cancer Foundation, Cure Cancer Australia Foundation, the Australian Cancer Research Foundation, the Petre Foundation, Young Garvan and the RT Hall Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- INK4 family
-
This family of CDK inhibitor proteins specifically prevent the activation of CDK4 and CDK6, generally by inhibiting cyclin D association.
- CIP and KIP family
-
This family of CDK inhibitor proteins bind cyclin–CDK complexes and are potent inhibitors of cyclin E–CDK2 and cyclin A–CDK2. They act as assembly factors for cyclin D–CDK4 and cyclin D–CDK6, but can also inhibit the activity of these kinases.
- DNA damage response
-
A global cellular response that halts cell cycle progression while damaged DNA is repaired, or that triggers cell death by apoptosis if the damage is too extensive for repair.
- Cyclin box
-
A domain that is characteristic of cyclins and has high sequence conservation across the cyclin family. It mediates cyclin–CDK binding.
- Oncogene addiction
-
Heightened dependency of cancer cells on specific oncogenes, so that, despite the presence of multiple genomic alterations, inactivation of a single oncogene can be sufficient to impair proliferation and survival.
- Phase I and II clinical trials
-
The first stages of clinical testing in humans. Phase I trials include tests of safety, tolerability, and pharmacokinetics; Phase II trials begin to assess efficacy.
Rights and permissions
About this article
Cite this article
Musgrove, E., Caldon, C., Barraclough, J. et al. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 11, 558–572 (2011). https://doi.org/10.1038/nrc3090
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3090
This article is cited by
-
G-quadruplexes promote the motility in MAZ phase-separated condensates to activate CCND1 expression and contribute to hepatocarcinogenesis
Nature Communications (2024)
-
SHR and SCR coordinate root patterning and growth early in the cell cycle
Nature (2024)
-
Co-delivery of artemisinin and metformin via PEGylated niosomal nanoparticles: potential anti-cancer effect in treatment of lung cancer cells
DARU Journal of Pharmaceutical Sciences (2024)
-
Functionalization of curcumin nanomedicines: a recent promising adaptation to maximize pharmacokinetic profile, specific cell internalization and anticancer efficacy against breast cancer
Journal of Nanobiotechnology (2023)
-
Calcineurin-mediated dephosphorylation enhances the stability and transactivation of c-Myc
Scientific Reports (2023)