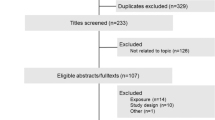
Key Points
-
Tea, made from the dried leaves of the plant Camellia sinensis, is the most popular beverage worldwide after water.
-
Tea and tea polyphenols have been shown to inhibit tumour formation and growth in different animal models for human cancer. The inhibition is associated with decreased cell proliferation, increased apoptosis and suppression of angiogenesis.
-
Tea polyphenols are antioxidants, but they can also generate reactive oxygen species. The major polyphenol from green tea, (−)-epigallocatechin-3-gallate, has been shown to bind directly to several receptors and signalling molecules, and to inhibit the functions of key receptors, kinases, proteinases and other enzymes.
-
Epidemiological studies, however, have not yielded conclusive results on the cancer-preventive effect of tea consumption in humans, possibly owing to different confounding factors. Some human cancer prevention trials with green tea polyphenol preparations have shown promising results.
-
Well-designed epidemiological studies and intervention trials are needed to evaluate the cancer-preventive activities of tea and tea polyphenols in humans.
-
Many issues raised and the experience gained from studies on tea and cancer prevention may be applicable to studies on other dietary constituents.
Abstract
Extracts of tea, especially green tea, and tea polyphenols have been shown to inhibit the formation and development of tumours at different organ sites in animal models. There is considerable evidence that tea polyphenols, in particular (−)-epigallocatechin-3-gallate, inhibit enzyme activities and signal transduction pathways, resulting in the suppression of cell proliferation and enhancement of apoptosis, as well as the inhibition of cell invasion, angiogenesis and metastasis. Here, we review these biological activities and existing data relating tea consumption to human cancer risk in an attempt to understand the potential use of tea for cancer prevention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Yang, C. S. & Wang, Z. Y. Tea and cancer. J. Natl Cancer Inst. 85, 1038–1049 (1993). This is the first comprehensive review of the cancer preventive activities of tea.
Bushman, J. L. Green tea and cancer in humans: a review of the literature. Nutr. Cancer 31, 151–159 (1998).
Yang, C. S., Maliakal, P. & Meng, X. Inhibition of carcinogenesis by tea. Annu. Rev. Pharmacol. Toxicol. 42, 25–54 (2002).
Hou, Z., Lambert, J. D., Chin, K. V. & Yang, C. S. Effects of tea polyphenols on signal transduction pathways related to cancer chemoprevention. Mutat. Res. 555, 3–19 (2004).
Lambert, J. D., Hong, J., Yang, G. Y., Liao, J. & Yang, C. S. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am. J. Clin. Nutr. 81, 284S–291S (2005).
Clark, J. & You, M. Chemoprevention of lung cancer by tea. Mol. Nutr. Food Res. 50, 144–151 (2006).
Khan, N., Afaq, F., Saleem, M., Ahmad, N. & Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 66, 2500–2505 (2006).
Wu, A. H. & Yu, M. C. Tea, hormone-related cancers and endogenous hormone levels. Mol. Nutr. Food Res. 50, 160–169 (2006).
Yang, C. S. et al. Possible mechanisms of the cancer-preventive activities of green tea. Mol. Nutr. Food Res. 50, 170–175 (2006).
Ju, J., Lu, G., Lambert, J. D. & Yang, C. S. Inhibition of carcinogenesis by tea constituents. Semin. Cancer Biol. 17, 395–402 (2007).
Balentine, D. A., Wiseman, S. A. & Bouwens, L. C. The chemistry of tea flavonoids. Crit. Rev. Food Sci. Nutr. 37, 693–704 (1997). This is a comprehensive review of the composition of different types of tea and the chemistry of tea constituents.
Valcic, S., Burr, J. A., Timmermann, B. N. & Liebler, D. C. Antioxidant chemistry of green tea catechins. New oxidation products of (−)-epigallocatechin gallate and (−)-epigallocatechin from their reactions with peroxyl radicals. Chem. Res. Toxicol. 13, 801–810 (2000).
Sang, S. M. et al. Theadibenzotropolone A, a new type pigment from enzymatic oxidation of (−)-epicatechin and (−)-epigallocatechin gallate and characterized from black tea using LC/MS/MS. Tetrahedron Lett. 43, 7129–7133 (2002).
Hou, Z. et al. Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 65, 8049–8056 (2005).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001).
Lee, M. J. et al. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol. Biomarkers Prev. 11, 1025–1032 (2002).
Chow, H. H. et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 9, 3312–3319 (2003). This article reports the detailed pharmacokinetics and safety of green tea polyphenols in humans.
Chow, H. H. et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin. Cancer Res. 11, 4627–4633 (2005).
Chen, L., Lee, M. J., Li, H. & Yang, C. S. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 25, 1045–1050 (1997).
Lambert, J. D. et al. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab. Dispos. 34, 8–11 (2006).
Yang, C. S., Sang, S., Lambert, J. D. & Lee, M. J. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol. Nutr. Food Res. 52 (Suppl. 1), 139–151 (2008).
Wang, Z. Y. et al. Inhibition of N-nitrosodiethylamine- and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorigenesis in A/J mice by green tea and black tea. Cancer Res. 52, 1943–1947 (1992). This is the first publication demonstrating the inhibition of lung carcinogenesis by green and black tea.
Xu, Y., Ho, C. T., Amin, S. G., Han, C. & Chung, F. L. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 52, 3875–3879 (1992). This is the first demonstration of the inhibition of lung tumorigenesis by EGCG and caffeine.
Yang, G. Y. et al. Black tea constituents, theaflavins, inhibit 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis in A/J mice. Carcinogenesis 18, 2361–2365 (1997).
Chung, F. L. et al. Inhibition of lung carcinogenesis by black tea in Fischer rats treated with a tobacco-specific carcinogen: caffeine as an important constituent. Cancer Res. 58, 4096–4101 (1998).
Mimoto, J. et al. (−)-Epigallocatechin gallate can prevent cisplatin-induced lung tumorigenesis in A/J mice. Carcinogenesis 21, 915–919 (2000).
Zhang, Z. et al. A germ-line p53 mutation accelerates pulmonary tumorigenesis: p53-independent efficacy of chemopreventive agents green tea or dexamethasone/myo-inositol and chemotherapeutic agents taxol or adriamycin. Cancer Res. 60, 901–907 (2000).
Liao, J. et al. Inhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green tea. Nutr. Cancer 48, 44–53 (2004).
Schuller, H. M., Porter, B., Riechert, A., Walker, K. & Schmoyer, R. Neuroendocrine lung carcinogenesis in hamsters is inhibited by green tea or theophylline while the development of adenocarcinomas is promoted: implications for chemoprevention in smokers. Lung Cancer 45, 11–18 (2004).
Lu, G. et al. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 66, 11494–11501 (2006).
Landau, J. M., Wang, Z. Y., Yang, G. Y., Ding, W. & Yang, C. S. Inhibition of spontaneous formation of lung tumors and rhabdomyosarcomas in A/J mice by black and green tea. Carcinogenesis 19, 501–507 (1998).
Sazuka, M., Murakami, S., Isemura, M., Satoh, K. & Nukiwa, T. Inhibitory effects of green tea infusion on in vitro invasion and in vivo metastasis of mouse lung carcinoma cells. Cancer Lett. 98, 27–31 (1995).
Lu, G. et al. Synergistic inhibition of lung tumorigenesis by a combination of green tea polyphenols and atorvastatin. Clin. Cancer Res. 14, 4981–4988 (2008).
Lu, Y. et al. A gene expression signature that can predict green tea exposure and chemopreventive efficacy of lung cancer in mice. Cancer Res. 66, 1956–1963 (2006).
Li, N. et al. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis 23, 1307–1313 (2002).
Wang, Z. Y. et al. Inhibition of N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in rats by green and black tea. Carcinogenesis 16, 2143–2148 (1995).
Yamane, T. et al. Inhibition of N-methyl-N'-nitro-N-nitrosoguanidine-induced carcinogenesis by (−)-epigallocatechin gallate in the rat glandular stomach. Cancer Res. 55, 2081–2084 (1995).
Murugan, R. S. et al. Modulatory effects of black tea polyphenols on oxidant–antioxidant profile and expression of proliferation, apoptosis, and angiogenesis-associated proteins in the rat forestomach carcinogenesis model. J. Gastroenterol. 42, 352–361 (2007).
Ju, J. et al. Inhibition of intestinal tumorigenesis in ApcMin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 65, 10623–10631 (2005).
Hao, X. et al. Inhibition of intestinal tumorigenesis in ApcMin/+ mice by green tea polyphenols (polyphenon E) and individual catechins. Nutr. Cancer 59, 62–69 (2007).
Xiao, H. et al. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis 29, 113–119 (2008).
Carter, O. et al. Comparison of white tea, green tea, epigallocatechin-3-gallate, and caffeine as inhibitors of PhIP-induced colonic aberrant crypts. Nutr. Cancer 58, 60–65 (2007).
Wang, R. et al. Protective versus promotional effects of white tea and caffeine on PhIP-induced tumorigenesis and β-catenin expression in the rat. Carcinogenesis 29, 834–839 (2008).
Liao, S., Umekita, Y., Guo, J., Kokontis, J. M. & Hiipakka, R. A. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 96, 239–243 (1995).
Gupta, S., Hastak, K., Ahmad, N., Lewin, J. S. & Mukhtar, H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc. Natl Acad. Sci. USA 98, 10350–10355 (2001). This is the first demonstration of the inhibition of prostate carcinogenesis in a mouse model by green tea polyphenols and related mechanisms.
Adhami, V. M., Siddiqui, I. A., Ahmad, N., Gupta, S. & Mukhtar, H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 64, 8715–8722 (2004).
Gao, Y. T. et al. Reduced risk of esophageal cancer associated with green tea consumption. J. Natl. Cancer Inst. 86, 855–858 (1994).
Fujiki, H., Suganuma, M., Imai, K. & Nakachi, K. Green tea: cancer preventive beverage and/or drug. Cancer Lett. 188, 9–13 (2002).
Zhang, M., Holman, C. D., Huang, J. P. & Xie, X. Green tea and the prevention of breast cancer: a case-control study in Southeast China. Carcinogenesis 28, 1074–1078 (2007).
Sun, C. L. et al. Urinary tea polyphenols in relation to gastric and esophageal cancers: a prospective study of men in Shanghai, China. Carcinogenesis 23, 1497–1503 (2002).
Yuan, J. M., Gao, Y. T., Yang, C. S. & Yu, M. C. Urinary biomarkers of tea polyphenols and risk of colorectal cancer in the Shanghai Cohort Study. Int. J. Cancer 120, 1344–1350 (2007).
Sasazuki, S., Inoue, M., Miura, T., Iwasaki, M. & Tsugane, S. Plasma tea polyphenols and gastric cancer risk: a case-control study nested in a large population-based prospective study in Japan. Cancer Epidemiol. Biomarkers Prev. 17, 343–351 (2008).
Wu, A. H., Yu, M. C., Tseng, C. C., Hankin, J. & Pike, M. C. Green tea and risk of breast cancer in Asian Americans. Int. J. Cancer 106, 574–579 (2003).
Wu, A. H., Tseng, C. C., Van Den Berg, D. & Yu, M. C. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 63, 7526–7529 (2003).
Bonner, M. R. et al. Green tea consumption, genetic susceptibility, PAH-rich smoky coal, and the risk of lung cancer. Mutat. Res. 582, 53–60 (2005).
Li, N., Sun, Z., Han, C. & Chen, J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proc. Soc. Exp. Biol. Med. 220, 218–224 (1999).
Ahn, W. S. et al. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur. J. Cancer Prev. 12, 383–390 (2003).
Bettuzzi, S. et al. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 66, 1234–1240 (2006). This is the first demonstration that green tea polyphenols prevented the progression of high-grade PIN to prostate cancer.
Shimizu, M. et al. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol. Biomarkers Prev. 17, 3020–3025 (2008).
Conney, A. H. et al. Stimulatory effect of oral administration of tea, coffee or caffeine on UVB-induced apoptosis in the epidermis of SKH-1 mice. Toxicol. Appl. Pharmacol. 224, 209–213 (2007).
Srividhya, R., Jyothilakshmi, V., Arulmathi, K., Senthilkumaran, V. & Kalaiselvi, P. Attenuation of senescence-induced oxidative exacerbations in aged rat brain by (−)-epigallocatechin-3-gallate. Int. J. Dev. Neurosci. 26, 217–223 (2008).
Senthil Kumaran, V., Arulmathi, K., Srividhya, R. & Kalaiselvi, P. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp. Gerontol. 43, 176–183 (2008).
Higdon, J. V. & Frei, B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 43, 89–143 (2003).
Inami, S. et al. Tea catechin consumption reduces circulating oxidized low-density lipoprotein. Int. Heart J. 48, 725–732 (2007).
Hsu, S. P. et al. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am. J. Clin. Nutr. 86, 1539–1547 (2007).
Hakim, I. A. et al. Effect of increased tea consumption on oxidative DNA damage among smokers: a randomized controlled study. J. Nutr. 133, 3303S–3309S (2003).
Schwartz, J. L., Baker, V., Larios, E. & Chung, F. L. Molecular and cellular effects of green tea on oral cells of smokers: a pilot study. Mol. Nutr. Food Res. 49, 43–51 (2005).
Yang, G. Y., Liao, J., Kim, K., Yurkow, E. J. & Yang, C. S. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 19, 611–616 (1998).
Na, H. K. & Surh, Y. J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 46, 1271–1278 (2008).
Shen, G. et al. Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/56J mice and C57BL/6J/Nrf2 (-/-) mice. Pharm. Res. 22, 1805–1820 (2005).
Chow, H. H. et al. Modulation of human glutathione S-transferases by polyphenon E intervention. Cancer Epidemiol. Biomarkers Prev. 16, 1662–1666 (2007).
Tang, L. et al. Modulation of aflatoxin biomarkers in human blood and urine by green tea polyphenols intervention. Carcinogenesis 29, 411–417 (2008).
Bonkovsky, H. L. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis). Ann. Intern. Med. 144, 68–71 (2006).
Lambert, J. D., Sang, S. & Yang, C. S. Possible controversy over dietary polyphenols: benefits vs risks. Chem. Res. Toxicol. 20, 583–585 (2007). This article discusses possible benefits and risks of taking green tea extract as a dietary supplement.
Tachibana, H., Koga, K., Fujimura, Y. & Yamada, K. A receptor for green tea polyphenol EGCG. Nature Struct. Mol. Biol. 11, 380–381 (2004). This is the first report of the identification of EGCG targets in a cell line.
Umeda, D., Yano, S., Yamada, K. & Tachibana, H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J. Biol. Chem. 283, 3050–3058 (2008).
Leone, M. et al. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 63, 8118–8121 (2003).
Ermakova, S. et al. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J. Biol. Chem. 280, 16882–16890 (2005). This is the first report of the identification of an EGCG target protein by affinity chromatography and molecular studies.
Li, M. et al. Direct inhibition of insulin-like growth factor-I receptor kinase activity by (−)-epigallocatechin-3-gallate regulates cell transformation. Cancer Epidemiol. Biomarkers Prev. 16, 598–605 (2007).
He, Z. et al. Fyn is a novel target of (−)-epigallocatechin gallate in the inhibition of JB6 C141 cell transformation. Mol. Carcinog. 47, 172–183 (2008).
Ermakova, S. P. et al. (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 66, 9260–9269 (2006).
Shim, J. H. et al. (−)-Epigallocatechin gallate regulates CD3-mediated T cell receptor signaling in leukemia through the inhibition of ZAP-70 kinase. J. Biol. Chem. 283, 28370–28379 (2008).
Mitsiades, C. S. et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 5, 221–230 (2004).
Shimizu, M., Deguchi, A., Hara, Y., Moriwaki, H. & Weinstein, I. B. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem. Biophys. Res. Commun. 334, 947–953 (2005).
Vittal, R. et al. Gene expression changes induced by green tea polyphenol (−)-epigallocatechin-3-gallate in human bronchial epithelial 21BES cells analyzed by DNA microarray. Mol. Cancer Ther. 3, 1091–1099 (2004).
Kuzuhara, T., Sei, Y., Yamaguchi, K., Suganuma, M. & Fujiki, H. DNA and RNA as new binding targets of green tea catechins. J. Biol. Chem. 281, 17446–17456 (2006).
Dong, Z., Ma, W., Huang, C. & Yang, C. S. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (−)-epigallocatechin gallate, and theaflavins. Cancer Res. 57, 4414–4419 (1997).
Chung, J. Y., Huang, C., Meng, X., Dong, Z. & Yang, C. S. Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in H-ras-transformed cells: structure–activity relationship and mechanisms involved. Cancer Res. 59, 4610–4617 (1999).
Chung, J. Y., Park, J. O., Phyu, H., Dong, Z. & Yang, C. S. Mechanisms of inhibition of the Ras–MAP kinase signaling pathway in 30.7b Ras 12 cells by tea polyphenols (−)-epigallocatechin-3-gallate and theaflavin-3,3′-digallate. FASEB J. 15, 2022–2024 (2001).
Liang, Y. C., Lin-Shiau, S. Y., Chen, C. F. & Lin, J. K. Inhibition of cyclin-dependent kinases 2 and 4 activities as well as induction of Cdk inhibitors p21 and p27 during growth arrest of human breast carcinoma cells by (−)-epigallocatechin-3-gallate. J. Cell Biochem. 75, 1–12 (1999).
Nam, S., Smith, D. M. & Dou, Q. P. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J. Biol. Chem. 276, 13322–13330 (2001).
Gupta, S., Ahmad, N., Nieminen, A. L. & Mukhtar, H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol. Appl. Pharmacol. 164, 82–90 (2000).
Gupta, S., Hussain, T. & Mukhtar, H. Molecular pathway for (−)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch. Biochem. Biophys. 410, 177–185 (2003).
Smith, D. M. et al. Synthetic analogs of green tea polyphenols as proteasome inhibitors. Mol. Med. 8, 382–392 (2002).
Garbisa, S. et al. Tumor invasion: molecular shears blunted by green tea. Nature Med. 5, 1216 (1999).
Garbisa, S. et al. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer 91, 822–832 (2001).
Annabi, B. et al. Green tea polyphenol (−)-epigallocatechin 3-gallate inhibits MMP-2 secretion and MT1-MMP-driven migration in glioblastoma cells. Biochim. Biophys. Acta 1542, 209–220 (2002).
Taniguchi, S. et al. Effect of (−)-epigallocatechin gallate, the main constituent of green tea, on lung metastasis with mouse B16 melanoma cell lines. Cancer Lett. 65, 51–54 (1992).
Fang, M. Z. et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 63, 7563–7570 (2003).
Navarro-Peran, E. et al. The antifolate activity of tea catechins. Cancer Res. 65, 2059–2064 (2005).
Shin, E. S. et al. Catechin gallates are NADP+-competitive inhibitors of glucose-6-phosphate dehydrogenase and other enzymes that employ NADP+ as a coenzyme. Bioorg. Med. Chem. 16, 3580–3586 (2008).
Ishii, T. et al. Covalent modification of proteins by green tea polyphenol (−)-epigallocatechin-3-gallate through autoxidation. Free Radic. Biol. Med. 45, 1384–1394 (2008).
Lo, H. W. & Hung, M. C. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br. J. Cancer 94, 184–188 (2006).
Liang, Y. C., Lin-shiau, S. Y., Chen, C. F. & Lin, J. K. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (−)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J. Cell Biochem. 67, 55–65 (1997).
Shimizu, M. et al. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 11, 2735–2746 (2005).
Adachi, S. et al. The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 67, 6493–6501 (2007).
Adachi, S. et al. (−)-Epigallocatechin gallate causes internalization of the epidermal growth factor receptor in human colon cancer cells. Carcinogenesis 29, 1986–1993 (2008).
Masuda, M. et al. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J. Exp. Ther. Oncol. 2, 350–359 (2002).
Peschard, P. & Park, M. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene 26, 1276–1285 (2007).
Birchmeier, C., Birchmeier, W., Gherardi, E. & Vande Woude, G. F. Met, metastasis, motility and more. Nature Rev. Mol. Cell Biol. 4, 915–925 (2003).
Bigelow, R. L. & Cardelli, J. A. The green tea catechins, (−)-Epigallocatechin-3-gallate (EGCG) and (−)-Epicatechin-3-gallate (ECG), inhibit HGF/Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene 25, 1922–1930 (2006).
Lim, Y. C. et al. (−)-Epigallocatechin-3-gallate (EGCG) inhibits HGF-induced invasion and metastasis in hypopharyngeal carcinoma cells. Cancer Lett. 271, 140–152 (2008).
Sartippour, M. R. et al. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J. Nutr. 132, 2307–2311 (2002).
Rodriguez, S. K. et al. Green tea catechin, epigallocatechin-3-gallate, inhibits vascular endothelial growth factor angiogenic signaling by disrupting the formation of a receptor complex. Int. J. Cancer 118, 1635–1644 (2006).
Zhu, B. H. et al. (−)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J. Gastroenterol. 13, 1162–1169 (2007).
Sukhthankar, M. et al. A green tea component suppresses posttranslational expression of basic fibroblast growth factor in colorectal cancer. Gastroenterology 134, 1972–1980 (2008).
Ciardiello, F. et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin. Cancer Res. 7, 1459–1465 (2001).
Weinstein, I. B. & Joe, A. Oncogene addiction. Cancer Res. 68, 3077–3080; discussion 3080 (2008).
Stoner, G. D. et al. Cancer prevention with freeze-dried berries and berry components. Semin. Cancer Biol. 17, 403–410 (2007).
Shumway, B. S. et al. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin. Cancer Res. 14, 2421–2430 (2008).
Acknowledgements
This work was supported by National Institutes of Health grants CA120915, CA122474 and CA133021.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
National Cancer Institute Drug Dictionary
FURTHER INFORMATION
Glossary
- Dietary recalls
-
A human dietary assessment procedure in which subjects are asked to recall their food consumptions over a certain time frame.
- Polyphenon E
-
A standard green tea polyphenol preparation containing 65% (−)-epigallocatechin-3-gallate.
- Atorvastatin
-
An inhibitor of 3-hydroxy-3-methyl-glutaryl CoA reductase and a commonly used cholesterol-lowering drug with the trade name Lipitor.
- Aflatoxin
-
A carcinogenic toxin produced by fungi that induces hepatic damage and leads to liver cancer.
- IC50
-
The concentration of a drug giving a 50% inhibition of the activity of a target enzyme.
- Lipid raft
-
A dynamic assembly of plasma membrane enriched in certain cholesterols, sphingolipids, saturated phospholipids and cell signalling proteins.
Rights and permissions
About this article
Cite this article
Yang, C., Wang, X., Lu, G. et al. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer 9, 429–439 (2009). https://doi.org/10.1038/nrc2641
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2641
This article is cited by
-
Regulation of gene expression by modulating microRNAs through Epigallocatechin-3-gallate in cancer
Molecular Biology Reports (2024)
-
Tea consumption and risk of bone health: an updated systematic review and meta-analysis
Journal of Bone and Mineral Metabolism (2024)
-
Metal-enriched HSP90 nanoinhibitor overcomes heat resistance in hyperthermic intraperitoneal chemotherapy used for peritoneal metastases
Molecular Cancer (2023)
-
Molecular identification of Lingyun Baihao wild and cultivated tea through genome-wide sequencing
Genetic Resources and Crop Evolution (2023)
-
Tea consumption and risk of lower respiratory tract infections: a two-sample mendelian randomization study
European Journal of Nutrition (2023)