Key Points
-
Chronic activation of the Ras-like (Ral) guanyl nucleotide-binding proteins, RALA and RALB, occurs in tumour-derived cell lines and tumour samples.
-
Depletion of RALA severely impairs the anchorage-independent proliferation of cancer cell lines, whereas RALB seems to be essential for the survival of a variety of tumour-derived cell lines.
-
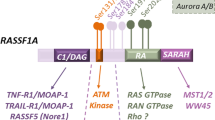
RALA is phosphorylated by Aurora kinase A and other, yet to be identified kinases. It is also a substrate of protein phosphatase 2A Aβ. Evidence indicates that dephosphorylation of RALA is a major mechanism by which PP2A Aβ normally restricts tumour progression.
-
The effects on tumorigenesis of well-characterised downstream effectors of Ral, such as the Rac-family GTPase-activating protein RLIP, the Y-box transcription factor ZO-1-associated nucleic acid-binding protein (ZONAB) and two subunits of the exocyst complex, SEC5 and EXO84, remain unclear. However, a number of relationships have been identified that might explain Ral-dependent modulation of cell proliferation and survival.
-
An important factor that might explain the occurrence of Ral activation in tumorigenesis is the RALB-specific contribution to cancer cell survival through activation of TANK-binding kinase 1 (TBK1). Chronic RALB activation restricts initiation of apoptotic programmes that are normally activated in the context of oncogenic stress.
-
Proteins such as TBK1 might prove to be good candidate targets for the development of cancer drugs with a large therapeutic window.
Abstract
A confluence of recent observations has indicted the Ras-family G-proteins RALA and RALB as key offenders in the subversion of core biological systems driving oncogenic transformation. Here, we will focus on current developments highlighting the pivotal contribution of Ral proteins to the regulatory framework supporting tumorigenesis, and evaluate mechanistic connections between Ral effector activation and generation of this framework.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chardin, P. & Tavitian, A. The ral gene: a new ras related gene isolated by the use of a synthetic probe. EMBO J. 5, 2203–2208 (1986).
Colicelli, J. Human RAS superfamily proteins and related GTPases. Sci. STKE 2004, RE13 (2004).
Feig, L. A., Urano, T. & Cantor, S. Evidence for a Ras/Ral signaling cascade. Trends Biochem. Sci. 21, 438–441 (1996). This review provides an in-depth survey of RalGTPase biology and biochemistry.
Feig, L. A. Ral-GTPases: approaching their 15 minutes of fame. Trends Cell Biol. 13, 419–425 (2003).
Camonis, J. H. & White, M. A. Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol. 15, 327–332 (2005).
Rangarajan, A., Hong, S. J., Gifford, A. & Weinberg, R. A. Species- and cell type-specific requirements for cellular transformation. Cancer Cell 6, 171–183 (2004).
Hamad, N. M. et al. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 16, 2045–2057 (2002).
Ward, Y. et al. Signal pathways which promote invasion and metastasis: critical and distinct contributions of extracellular signal-regulated kinase and Ral-specific guanine exchange factor pathways. Mol. Cell Biol. 21, 5958–5969 (2001).
Lim, K. H. et al. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell 7, 533–545 (2005).
Lim, K. H. et al. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr. Biol. 16, 2385–2394 (2006). This study uses short hairpin RNA-mediated loss of function to examine the contribution of RALA and RALB to orthotopic xenograft tumour formation and metastasis.
Chien, Y. et al. RalB GTPase-mediated activation of the IκB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell 127, 157–170 (2006). This study establishes mechanistic relationships coupling RALB activation to cancer cell survival.
Chien, Y. & White, M. A. RAL GTPases are linchpin modulators of human tumour-cell proliferation and survival. EMBO Rep. 4, 800–806 (2003).
Falsetti, S. C. et al. Geranylgeranyltransferase I inhibitors target RalB to inhibit anchorage-dependent growth and induce apoptosis, and RalA to inhibit anchorage-independent growth. Mol. Cell Biol. 27, 8003–8014 (2007).
Sablina, A. A. et al. The tumor suppressor PP2A Aβ regulates the RalA GTPase. Cell 129, 969–982 (2007). This study establishes phospho-RALA as a central PP2A tumour suppression target.
Takagi, Y. et al. Alterations of the PPP2R1B gene located at 11q23 in human colorectal cancers. Gut 47, 268–271 (2000).
Tamaki, M., Goi, T., Hirono, Y., Katayama, K. & Yamaguchi, A. PPP2R1B gene alterations inhibit interaction of PP2A-Aβ and PP2A- proteins in colorectal cancers. Oncol. Rep. 11, 655–659 (2004).
Wang, S. S. et al. Alterations of the PPP2R1B gene in human lung and colon cancer. Science 282, 284–287 (1998).
Calin, G. A. et al. Low frequency of alterations of the α (PPP2R1A) and β (PPP2R1B) isoforms of the subunit A of the serine-threonine phosphatase 2A in human neoplasms. Oncogene 19, 1191–1195 (2000).
Baysal, B. E. et al. A high-resolution integrated map spanning the SDHD gene at 11q23: a 1.1-Mb BAC contig, a partial transcript map and 15 new repeat polymorphisms in a tumour-suppressor region. Eur. J. Hum. Genet. 9, 121–129 (2001).
Wu, J. C. et al. Identification of V23RalA-Ser194 as a critical mediator for Aurora-A-induced cellular motility and transformation by small pool expression screening. J. Biol. Chem. 280, 9013–9022 (2005).
Bivona, T. G. et al. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol. Cell 21, 481–493 (2006).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Bissell, M. J. & Radisky, D. Putting tumours in context. Nature Rev. Cancer 1, 46–54 (2001).
Yin, J. et al. Activation of the RalGEF/Ral pathway promotes prostate cancer metastasis to bone. Mol. Cell Biol. 27, 7538–7550 (2007).
Oxford, G., Smith, S. C., Hampton, G. & Theodorescu, D. Expression profiling of Ral-depleted bladder cancer cells identifies RREB-1 as a novel transcriptional Ral effector. Oncogene 26, 7143–7152 (2007).
Smith, S. C. et al. The metastasis-associated gene CD24 is regulated by Ral GTPase and is a mediator of cell proliferation and survival in human cancer. Cancer Res. 66, 1917–1922 (2006).
Gonzalez-Garcia, A. et al. RalGDS is required for tumor formation in a model of skin carcinogenesis. Cancer Cell 7, 219–226 (2005). This study demonstrates that Ral activation promotes spontaneous tumour initiation and progression in vivo.
Cantor, S. B., Urano, T. & Feig, L. A. Identification and characterization of Ral-binding protein 1, a potential downstream target of Ral GTPases. Mol. Cell. Biol. 15, 4578–4584 (1995).
Jullien-Flores, V. et al. Bridging Ral GTPase to Rho pathways. RLIP76, a Ral effector with CDC42/Rac GTPase-activating protein activity. J. Biol. Chem. 270, 22473–22477 (1995).
Frankel, P. et al. RalA interacts with ZONAB in a cell density-dependent manner and regulates its transcriptional activity. EMBO J. 24, 54–62 (2005).
Moskalenko, S. et al. The exocyst is a Ral effector complex. Nature Cell Biol. 4, 66–72 (2002).
Sugihara, K. et al. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nature Cell Biol. 4, 73–78 (2002).
Moskalenko, S. et al. Ral GTPases regulate exocyst assembly through dual subunit interactions. J. Biol. Chem. 278, 51743–51748 (2003).
Jullien-Flores, V. et al. RLIP76, an effector of the GTPase Ral, interacts with the AP2 complex: involvement of the Ral pathway in receptor endocytosis. J. Cell Sci. 113, 2837–2844 (2000).
Nakashima, S. et al. Small G. protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. EMBO J. 18, 3629–3642 (1999).
Singhal, S. S., Awasthi, Y. C. & Awasthi, S. Regression of melanoma in a murine model by RLIP76 depletion. Cancer Res. 66, 2354–2360 (2006).
Singhal, S. S. et al. Regression of lung and colon cancer xenografts by depleting or inhibiting RLIP76 (Ral-binding protein 1). Cancer Res. 67, 4382–4389 (2007).
Panner, A. et al. mTOR-independent translational control of the extrinsic cell death pathway by RalA. Mol. Cell. Biol. 26, 7345–7357 (2006).
de Ruiter, N. D., Wolthuis, R. M., van Dam, H., Burgering, B. M. & Bos, J. L. Ras-dependent regulation of c-Jun phosphorylation is mediated by the Ral guanine nucleotide exchange factor–Ral pathway. Mol. Cell. Biol. 20, 8480–8488 (2000).
Henry, D. O. et al. Ral GTPases contribute to regulation of cyclin D1 through activation of NF-kB. Mol. Cell. Biol. 20, 8084–8092 (2000).
Chang, L. et al. The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIP(L) turnover. Cell 124, 601–613 (2006).
Micheau, O., Lens, S., Gaide, O., Alevizopoulos, K. & Tschopp, J. NF-κB signals induce the expression of c-FLIP. Mol. Cell Biol. 21, 5299–5305 (2001).
Balda, M. S. & Matter, K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 19, 2024–2033 (2000).
Kavanagh, E. et al. Functional interaction between the ZO-1-interacting transcription factor ZONAB/DbpA and the RNA processing factor symplekin. J. Cell Sci. 119, 5098–5105 (2006).
Balda, M. S., Garrett, M. D. & Matter, K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 160, 423–432 (2003).
Sourisseau, T. et al. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol. Cell Biol. 26, 2387–2398 (2006).
Guo, W., Sacher, M., Barrowman, J., Ferro-Novick, S. & Novick, P. Protein complexes in transport vesicle targeting. Trends Cell Biol. 10, 251–255. (2000).
Hsu, S. C., TerBush, D., Abraham, M. & Guo, W. The exocyst complex in polarized exocytosis. Int. Rev. Cytol. 233, 243–265 (2004).
Inoue, M., Chang, L., Hwang, J., Chiang, S. H. & Saltiel, A. R. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature 422, 629–633 (2003).
Rosse, C. et al. RalB mobilizes the exocyst to drive cell migration. Mol. Cell Biol. 26, 727–734 (2006).
Chen, X. W., Inoue, M., Hsu, S. C. & Saltiel, A. R. RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. J. Biol. Chem. 281, 38609–38616 (2006).
Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430, 257–263 (2004).
Hiscott, J. Another detour on the Toll road to the interferon antiviral response. Nature Struct. Mol. Biol. 11, 1028–1030 (2004).
McWhirter, S. M., Tenoever, B. R. & Maniatis, T. Connecting mitochondria and innate immunity. Cell 122, 645–647 (2005).
Lee, H. K., Dunzendorfer, S., Soldau, K. & Tobias, P. S. Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity 24, 153–163 (2006).
Kawai, T. et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nature Immunol. 6, 981–988 (2005).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Fitzgerald, K. A. et al. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nature Immunol. 4, 491–496 (2003).
Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 (2003).
McWhirter, S. M. et al. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl Acad. Sci. USA 101, 233–238 (2004).
Buss, H. et al. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-κB at serine 536 is mediated by multiple protein kinases including IκB kinase (IKK)-α, IKKβ, IKKε, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J. Biol. Chem. 279, 55633–55643 (2004).
Hacker, H. & Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, RE13 (2006).
Boehm, J. S. et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell 129, 1065–1079 (2007).
Mantovani, A. & Balkwill, F. RalB signaling: a bridge between inflammation and cancer. Cell 127, 42–44 (2006).
Korherr, C. et al. Identification of proangiogenic genes and pathways by high-throughput functional genomics: TBK1 and the IRF3 pathway. Proc. Natl Acad. Sci. USA 103, 4240–4245 (2006).
Buess, M. et al. Characterization of heterotypic interaction effects in vitro to deconvolute global gene expression profiles in cancer. Genome Biol. 8, R191 (2007).
Kuranaga, E. et al. Drosophila IKK-related kinase regulates nonapoptotic function of caspases via degradation of IAPs. Cell 126, 583–596 (2006).
Adli, M. & Baldwin, A. S. IKK-i/IKKε controls constitutive, cancer cell-associated NF-κB activity via regulation of Ser-536 p65/RelA phosphorylation. J. Biol. Chem. 281, 26976–26984 (2006).
Downward, J. Signal transduction. Prelude to an anniversary for the RAS oncogene. Science 314, 433–434 (2006).
Smith, S. C. et al. Expression of ral GTPases, their effectors, and activators in human bladder cancer. Clin. Cancer Res. 13, 3803–3813 (2007).
Frech, M., Schlichting, I., Wittinghofer, A. & Chardin, P. Guanine nucleotide binding properties of the mammalian RalA protein produced in Escherichia coli. J. Biol. Chem. 265, 6353–6359 (1990).
Emkey, R., Freedman, S. & Feig, L. A. Characterization of a GTPase-activating protein for the Ras-related Ral protein. J. Biol. Chem. 266, 9703–9706 (1991).
Leonardi, P. et al. Human rgr: transforming activity and alteration in T-cell malignancies. Oncogene 21, 5108–5116 (2002).
Jimenez, M. et al. The Rgr oncogene induces tumorigenesis in transgenic mice. Cancer Res. 64, 6041–6049 (2004).
Tian, X., Rusanescu, G., Hou, W., Schaffhausen, B. & Feig, L. A. PDK1 mediates growth factor-induced Ral-GEF activation by a kinase-independent mechanism. EMBO J. 21, 1327–1338 (2002).
Yoshizaki, H., Mochizuki, N., Gotoh, Y. & Matsuda, M. Akt–PDK1 complex mediates epidermal growth factor-induced membrane protrusion through Ral activation. Mol. Biol. Cell 18, 119–128 (2007).
Ceriani, M. et al. Functional analysis of RalGPS2, a murine guanine nucleotide exchange factor for RalA GTPase. Exp. Cell Res. 313, 2293–2307 (2007).
Tartaglia, M. et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nature Genet. 39, 75–79 (2007).
Sjoblom, T. et al. The consensus coding sequences of human breast and colorectal cancers. Science 314, 268–274 (2006).
Bamford, S. et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br. J. Cancer 91, 355–358 (2004).
Greenman, C. et al. Patterns of somatic mutation in human cancer genomes. Nature 446, 153–158 (2007).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Glossary
- Geranylgeranyltransferase 1
-
(GGTase 1). One of the three enzymes in the prenyltransferase group. GGTase 1 adds a 20-carbon isoprenoid called a geranylgeranyl group to proteins bearing a CaaX motif (a four amino-acid sequence at the C terminus of a protein).
- Farnesylation
-
After translation, the Ras proteins undergo four modification steps: isoprenylation, proteolysis, methylation and palmitoylation. Isoprenylation involves the enzyme farnesyltransferase, which transfers a farnesyl group from farnesyl pyrophosphate.
- Endomembrane domains
-
Internal membranes of the cell such as the nuclear membrane, Golgi and endoplasmic reticulum.
- Exocyst complex
-
The exocyst is a large complex of proteins required for polarised exocytosis in eukaryotic cells. It seems to function primarily as a tether, directing secretory vesicles to specific sites on the plasma membrane.
- Coated-pit endocytosis
-
The coated pit is a specialized region of the membrane that is coated with clathrin (for stability, to aid the transport process). The coated pit forms a coated vesicle and then loses its clathrin coat.
- Clathrin adaptor proteins
-
Proteins that recruit clathrin to membranes and concentrate specific transmembrane proteins in clathrin-coated areas of the membrane.
- Apoptosome
-
A caspase-activating complex that is formed when cytochrome c is released from mitochondria. It initiates oligomerization of APAF1, which binds procaspase 9 and thereby initiates the caspase cascade that leads to programmed cell death.
- Innate immune signalling
-
The innate immune system includes phagocytes, natural killer cells, the complement system and other non-specific components. It protects against infections using mechanisms that exist before infection, providing a rapid response to microbes that is essentially the same regardless of the type of infection.
- Myristoylated
-
Refers to the accession of fatty moieties that allow association with the inner layer of the plasma membrane.
- X-linked lymphoproliferative syndrome
-
This is a rare immunodeficiency disease characterized by fatal or near-fatal Epstein–Barr virus-induced infectious mononucleosis in childhood, subsequent hypogammaglobulinaemia and a markedly increased risk of lymphoma or other lymphoproliferative diseases.
Rights and permissions
About this article
Cite this article
Bodemann, B., White, M. Ral GTPases and cancer: linchpin support of the tumorigenic platform. Nat Rev Cancer 8, 133–140 (2008). https://doi.org/10.1038/nrc2296
Issue Date:
DOI: https://doi.org/10.1038/nrc2296
This article is cited by
-
The exocyst complex in neurological disorders
Human Genetics (2023)
-
Regulation of Ras p21 and RalA GTPases activity by quinine in mammary epithelial cells
Molecular and Cellular Biochemistry (2023)
-
The role of ral signaling and post translational modifications (PTMs) of Ras in cancer
Genome Instability & Disease (2022)
-
κB-Ras and Ral GTPases regulate acinar to ductal metaplasia during pancreatic adenocarcinoma development and pancreatitis
Nature Communications (2020)
-
RALB GTPase: a critical regulator of DR5 expression and TRAIL sensitivity in KRAS mutant colorectal cancer
Cell Death & Disease (2020)