Abstract
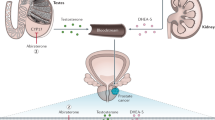
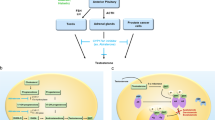
Prostate cancer is the most commonly diagnosed cancer and the second most common cause of cancer-related death in men, and benign prostatic hyperplasia is the most common benign condition known to occur in ageing men. Oestrogen has been implicated in the development of prostate cancer, and offers a promising new avenue for treatment. Despite this, the role of oestrogens in the prostate is complex. This Perspective presents a rationale for a targeted approach for the treatment of prostate disease through the use of selective oestrogen-receptor modulators in conjunction with contemporary androgen-ablation therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cunha, G. et al. The endocrinology and developmental biology of the prostate. Endocrine Rev. 8, 338–362 (1987).
Wilding, G. The importance of steroid hormones in prostate cancer. Cancer Surv. 14, 113–130 (1992).
Huggins, C. & Hodges, C. V. Studies on prostatic cancer. The effect of castration, of estrogen and of androgen interaction on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 293–297 (1941).
Berry, S. J., Coffey, D. S., Walsh, P. C. & Ewing, L. L. The development of human benign prostatic hyperplasia with age. J. Urol. 132, 474–479. (1984).
Ekman, P. BPH epidemiology and risk factors. Prostate Suppl. 2, 23–31 (1989).
Partin, A. W. et al. Concordance rates for benign prostatic disease among twins suggest hereditary influence. Urology 44, 646–650 (1994).
Leav, I., Merk, F. B., Kwan, P. W. & Ho, S. M. Androgen-supported estrogen-enhanced epithelial proliferation in the prostates of intact Noble rats. Prostate 15, 23–40 (1989).
Leav, I. et al. Comparative studies of the estrogen receptors β and α and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am. J. Pathol. 159, 79–92 (2001).
Cunha, G. R., Wang, Y. Z., Hayward, S. W. & Risbridger, G. P. Estrogenic effects on prostatic differentiation and carcinogenesis. Reprod. Fertil. Dev. 13, 285–296 (2001).
Ross, R. K. et al. 5-α-reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet 339, 887–889 (1992).
Bosland, M. C. The role of steroid hormones in prostate carcinogenesis. J. Natl Cancer Inst. Monogr. 39–66 (2000).
Hill, P., Garbaczewski, L. & Walker, A. R. Age, environmental factors and prostatic cancer. Med. Hypotheses 14, 29–39 (1984).
Jarred, R. et al. Induction of apoptosis in low grade human prostate carcinoma by red clover-derived dietary isoflavones. Cancer Res. (2002).
Bylund, A. et al. Rye bran and soy protein delay growth and increase apoptosis of human LNCaP prostate adenocarcinoma in nude mice. Prostate 42, 304–314. (2000).
Landstrom, M. et al. Estrogen induces apoptosis in a rat prostatic adenocarcinoma: association with an increased expression of TGF-beta 1 and its type-I and type-II receptors. Int. J. Cancer 67, 573–579 (1996).
Peterson, G. & Barnes, S. Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate 22, 335–345 (1993).
Wang, J., Eltoum, I. E. & Lamartiniere, C. A. Dietary genistein suppresses chemically induced prostate cancer in Lobund-Wistar rats. Cancer Lett. 186, 11–18 (2002).
Adlercreutz, H. Epidemiology of phytoestrogens. Baillieres Clin. Endocrinol. Metab. 12, 605–623. (1998).
Adlercreutz, H., Markkanen, H. & Watanabe, S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet 342, 1209–1210 (1993).
Morton, M., Turkes, A., Denis, L. & Griffiths, K. Can dietary factors influence prostatic disease? BJU Int. 84, 594–554 (1999).
Morton, M. S. et al. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United Kingdom. Prostate 32, 122–128 (1997).
Ellem, S. J., Schmitt, J. F., Pedersen, J. S., Frydenberg, M. & Risbridger, G. P. Local aromatase expression in human prostate is altered in malignancy. J. Clin. Endocrinol. Metab. 89, 2434–2441 (2004).
Hiramatsu, M. et al. Aromatase in hyperplasia and carcinoma of the human prostate. The Prostate 31, 118–124 (1997).
Harada, N., Utsumi, T. & Takagi, Y. Tissue-specific expression of the human aromatase cytochrome P-450 gene by alternative use of multiple exons 1 and promoters, and switching of tissue-specific exons 1 in carcinogenesis. Proc. Natl Acad. Sci. USA 90, 11312–11316. (1993).
Jones, M. E., Boon, W. C., Proietto, J. & Simpson, E. R. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol. Metab. 17, 55–64 (2006).
Vermeulen, A., Rubens, R. & Verdonck, L. Testosterone secretion and metabolism in male senescence. J. Clin. Endocrinol. Metab. 34, 730–735 (1972).
Vermeulen, A., Kaufman, J. M., Goemaere, S. & van Pottelberg, I. Estradiol in elderly men. Aging Male 5, 98–102 (2002).
Baulieu, E. E. Androgens and aging men. Mol. Cell Endocrinol. 198, 41–49 (2002).
Ho, S. M. & Roy, D. Sex hormone-induced nuclear DNA damage and lipid peroxidation in the dorsolateral prostates of Noble rats. Cancer Lett. 84, 155–162 (1994).
Prins, G. S. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology 130, 3703–3714 (1992).
McPherson, S. et al. Elevated androgens and prolactin in aromatase deficient (ArKO) mice cause enlargement but not malignancy of the prostate gland. Endocrinology 142, 2458–2467 (2001).
Rohrmann, S. et al. Serum estrogen, but not testosterone levels differ between black and white men in a nationally representative sample of Americans. J. Clin. Endocrinol. Metab. 24 April 2007 (epub ahead of print)
de Jong, F. H. et al. Peripheral hormone levels in controls and patients with prostatic cancer or benign prostatic hyperplasia: results from the Dutch-Japanese case-control study. Cancer Res. 51, 3445–3450 (1991).
Fishman, J. & Goto, J. Mechanism of estrogen biosynthesis. Participation of multiple enzyme sites in placental aromatase hydroxylations. J. Biol. Chem. 256, 4466–4471 (1981).
Akhtar, M., Calder, M. R., Corina, D. L. & Wright, J. N. Mechanistic studies on C-19 demethylation in oestrogen biosynthesis. Biochem. J. 201, 569–580 (1982).
Simpson, E. R., Mahendroo, M. S., Nichols, J. E. & Bulun, S. E. Aromatase gene expression in adipose tissue: relationship to breast cancer. Int. J. Fertil. Menopausal Stud. 39, 75–83 (1994).
Kruit, W. H., Stoter, G. & Klijn, J. G. Effect of combination therapy with aminoglutethimide and hydrocortisone on prostate-specific antigen response in metastatic prostate cancer refractory to standard endocrine therapy. Anticancer Drugs 15, 843–847 (2004).
Ponder, B. A. et al. Response to aminoglutethimide and cortisone acetate in advanced prostatic cancer. Br. J. Cancer 50, 757–763 (1984).
Rostom, A. Y. et al. Aminoglutethimide therapy for advanced carcinoma of the prostate. Br. J. Urol. 54, 552–555 (1982).
Smith, M. R. et al. Selective aromatase inhibition for patients with androgen-independent prostate carcinoma. Cancer 95, 1864–1868 (2002).
Santen, R. J. et al. Use of the aromatase inhibitor anastrozole in the treatment of patients with advanced prostate carcinoma. Cancer 92, 2095–2101 (2001).
Price, D. et al. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: results of a double-blind, placebo controlled, phase IIB clinical trial. J. Urol. 176, 965–970 (2006).
Smith, E. P. et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 331, 1056–1061 (1994).
Carani, C. et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N. Engl. J. Med. 337, 91–95 (1997).
Olsen, N. J. & Kovacs, W. J. Gonadal steroids and immunity. Endocr. Rev. 17, 369–384 (1996).
Mendelsohn, M. E. & Karas, R. H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 340, 1801–1811 (1999).
Risbridger, G. et al. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Dev. Biol. 229, 432–442. (2001).
Pylkkanen, L., Santti, R., Newbold, R. & McLachlan, J. A. Regional differences in the prostate of the neonatally estrogenized mouse. Prostate 18, 117–129 (1991).
Prins, G. in Prostate: Basic and Clinical Aspects (ed. Naz, R.) 245–263 (CRC Press, New York, 1997).
Naslund, M. J., Strandberg, J. D. & Coffey, D. S. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J. Urol. 140, 1049–1053 (1988).
Stoker, T. E., Robinette, C. L. & Cooper, R. L. Perinatal exposure to estrogenic compounds and the subsequent effects on the prostate of the adult rat: evaluation of inflammation in the ventral and lateral lobes. Reprod. Toxicol. 13, 463–472 (1999).
Bianco, J. J., Handelsman, D. J., Pedersen, J. S. & Risbridger, G. P. Direct response of the murine prostate gland and seminal vesicles to estradiol. Endocrinology 143, 4922–4933 (2002).
Bianco, J. J., McPherson, S. J., Wang, H., Prins, G. S. & Risbridger, G. P. Transient neonatal estrogen exposure to estrogen deficient mice (Aromatase knockout) reduces prostate weight and induces inflammation in late life. Am. J. Pathol. 168, 1869–1878 (2006).
Prins, G. S. et al. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor α: studies with αERKO and βERKO mice. Cancer Res. 61, 6089–6097 (2001).
Nickel, J. C. Prostatic inflammation in benign prostatic hyperplasia- the third component? Can. J. Urol. 1, 1–4 (1994).
Kramer, G. & Marberger, M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr. Opin. Urol. 16, 25–29 (2006).
Gleason, P. E. et al. Platelet derived growth factor (PDGF), androgens and inflammation: possible etiologic factors in the development of prostatic hyperplasia. J. Urol. 149, 1586–1592 (1993).
De Marzo, A. M., Marchi, V. L., Epstein, J. I. & Nelson, W. G. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am. J. Pathol. 155, 1985–1992 (1999).
Palapattu, G. S. et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis 26, 1170–1181 (2004).
Nelson, W. G., De Marzo, A. M., DeWeese, T. L. & Isaacs, W. B. The role of inflammation in the pathogenesis of prostate cancer. J. Urol. 172, S6–S11 (2004).
De Marzo, A. M. et al. Inflammation in prostate carcinogenesis. Nature Rev. Cancer 7, 256–269 (2007).
Ho, S. et al. Induction of atypical hyperplasia, apoptosis, and type II estrogen-binding sites in the ventral prostates of Noble rats treated with testosterone and pharmacologic doses of estradiol-17 β. Lab. Invest. 73, 356–365 (1995).
Gingrich, J. R. et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 56, 4096–4102 (1996).
Raghow, S., Hooshdaran, M. Z., Katiyar, S. & Steiner, M. S. Toremifene prevents prostate cancer in the transgenic adenocarcinoma of mouse prostate model. Cancer Res. 62, 1370–1376 (2002).
Cassidy, A. Potential risks and benefits of phytoestrogen-rich diets. Int. J. Vitam. Nutr. Res. 73, 120–126 (2003).
Sirtori, C. R., Arnoldi, A. & Johnson, S. K. Phytoestro-gens: end of a tale? Ann. Med. 37, 423–438 (2005).
Severson, R. K., Nomura, A. M., Grove, J. S. & Stemmermann, G. N. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 49, 1857–1860 (1989).
Adlercreutz, H. et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am. J. Clin. Nutr. 54, 1093–1100 (1991).
Harris, H. A. Estrogen receptor-β: Recent lessons from in vivo studies. Mol. Endocrinol. 21, 1–13 (2007).
Kuiper, G. G., Enmark, E., Pelto-Huikko, M., Nilsson, S. & Gustafsson, J. A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl Acad. Sci. USA 93, 5925–5930 (1996).
Weihua, Z., Warner, M. & Gustafsson, J. Estrogen receptor β in the prostate. Mol. Cell. Endocrinol. 193, 1 (2002).
Zhu, X. et al. Dynamic regulation of estrogen receptor-β expression by DNA methylation during prostate cancer development and metastasis. Am. J. Pathol. 164, 2003–2012 (2004).
Cheng, J., Lee, E. J., Madison, L. D. & Lazennec, G. Expression of estrogen receptor beta in prostate carcinoma cells inhibits invasion and proliferation and triggers apoptosis. FEBS Lett. 566, 169–172 (2004).
Weihua, Z. et al. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc. Natl Acad. Sci. USA 98, 6330–6335 (2001).
Krege, J. H. et al. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc. Natl Acad. Sci. USA 95, 15677–15682 (1998).
Dupont, S. et al. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127, 4277–4291 (2000).
Shughrue, P. J., Askew, G. R., Dellovade, T. L. & Merchenthaler, I. Estrogen-binding sites and their functional capacity in estrogen receptor double knockout mouse brain. Endocrinology 143, 1643–1650 (2002).
Imamov, O. et al. Estrogen receptor β regulates epithelial cellular differentiation in the mouse ventral prostate. Proc. Natl Acad. Sci. USA 101, 9375–9380 (2004).
Weihua, Z., Lathe, R., Warner, M. & Gustafsson, J. A. An endocrine pathway in the prostate, ERβ, AR, 5α-androstane-3β, 17β-diol, and CYP7B1, regulates prostate growth. Proc. Natl Acad. Sci. USA 99, 13589–13594 (2002).
Imamov, O., Lopatkin, N. A. & Gustafsson, J. A. Estrogen receptor β in prostate cancer. N. Engl. J. Med. 351, 2773–2774 (2004).
Thompson, I. M. et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 349, 215–224 (2003).
McPherson, S. J. et al. Essential role for estrogen receptor β in stromal-epithelial regulation of prostatic hyperplasia. Endocrinology 148, 566–574 (2006).
Thompson, T. C., Cunha, G. R., Shannon, J. M. & Chung, L. W. Androgen-induced biochemical responses in epithelium lacking androgen receptors: characterization of androgen receptors in the mesenchymal derivative of urogenital sinus. J. Steroid Biochem. 25, 627–634 (1986).
Cunha, G. R. & Donjacour, A. Stromal-epithelial interactions in normal and abnormal prostatic development. Prog. Clin. Biol. Res. 239, 251–272 (1987).
Cunha, G. R., Young, P., Higgins, S. J. & Cooke, P. S. Neonatal seminal vesicle mesenchyme induces a new morphological and functional phenotype in the epithelia of adult ureter and ductus deferens. Development 111, 145–158 (1991).
Matthews, J. & Gustafsson, J. A. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol. Interv. 3, 281–292 (2003).
Wang, Z. et al. A variant of estrogen receptor-α, hER-α 36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc. Natl Acad. Sci. USA 103, 9063–9068 (2006).
Levin, E. R. Cell localization, physiology, and nongenomic actions of estrogen receptors. J. Appl. Physiol. 91, 1860–1867 (2001).
Bjornstrom, L. & Sjoberg, M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 19, 833–842 (2005).
Acknowledgements
The authors thank S. McPherson, R. Santen and F. Gardiner for their critical review of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Ellem, S., Risbridger, G. Treating prostate cancer: a rationale for targeting local oestrogens. Nat Rev Cancer 7, 621–627 (2007). https://doi.org/10.1038/nrc2174
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2174
This article is cited by
-
Obesity-associated inflammation induces androgenic to estrogenic switch in the prostate gland
Prostate Cancer and Prostatic Diseases (2020)
-
A synthesis strategy for tetracyclic terpenoids leads to agonists of ERβ
Nature Communications (2019)
-
Oestrogen receptor beta isoform expression in sporadic colorectal cancer, familial adenomatous polyposis and progressive stages of colorectal cancer
BMC Cancer (2017)
-
Enhancing active surveillance of prostate cancer: the potential of exercise medicine
Nature Reviews Urology (2016)
-
Selective Estrogen Receptor β Agonist LY500307 as a Novel Therapeutic Agent for Glioblastoma
Scientific Reports (2016)