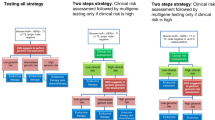
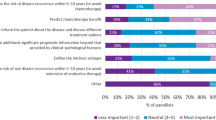
Abstract
Each year, over 200,000 people are diagnosed with breast cancer in the United States. Although the use of biomarkers has the potential to guide preventive interventions and improve survival and quality of life, there have been few successes and many disappointments. In November 2005, the National Breast Cancer Coalition Fund convened a conference aimed at developing a patient-centred, strategic approach to breast cancer biomarker research. The consumers, clinicians, researchers, industry representatives and US regulators who served on the consensus panel developed a set of principles and recommendations to guide the field and ensure that biomarker research results in clinically important applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
World Health Organization. Cancer. WHO [online], (2006).
American Cancer Society. Cancer Facts and Figures 2007 (American Cancer Society, Atlanta, Georgia, 2007).
Mincey, B. A. Genetics and the management of women at high risk for breast cancer. Oncologist 8, 466–473 (2003).
US Food and Drug Administration. FDA Clears Breast Cancer Specific Molecular Prognostic Test. FDA News [online], (2007).
McShane L. M. et al. Reporting recommendations for tumor MARKer prognostic studies (REMARK). Nature Clin. Pract. Oncol. 2, 416–422 (2005).
McShane L. M. et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Brit. J. Cancer 93, 387–391 (2005).
McShane L. M. et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Euro. J. Cancer 41, 1690–1696 (2005).
McShane L. M. et al. Reporting recommendations for tumor marker prognostic studies. J. Clin. Oncology 23, 9067–9072 (2005).
McShane L. M. et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J. Natl Cancer Inst. 97, 1180–1184 (2005).
Moher, D., Schulz, K. F. & Altman, D. CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 285, 1987–1991 (2001).
Altman, D. G. for the CONSORT Group. Endorsement of the CONSORT statement by high impact medical journals: survey of instructions for authors. BMJ 330, 1056–1057 (2005).
De Angelis C. et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N. Engl. J. Med. 351, 1250–1251 (2004).
De Angelis C. D. et al. Is this clinical trial fully registered — a statement from the International Committee of Medical Journal Editors. N. Engl. J. Med. 352, 2436–2438 (2005).
Bast, R. C. Jr, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J. Clin. Oncol. 19, 1865–1878 (2001).
Wolff, A. C. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 25, 118–145 (2007).
van de Vijver, M. J. et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347, 1999–2009 (2002).
Paik S. et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 351, 2817–2826 (2004).
US Food and Drug Administration. Draft Guidance for Industry, Clinical Laboratories, and FDA Staff — In Vitro Diagnostic Multivariate Index Assays. FDA [online], (2006).
Bellman, P., Havens, C., Bertolucco, Y. & Streeter, B. Facilitating physician access to medical reference information. Permanente J. 9, 27–32 (2005)
The National Breast Cancer Coalition. A proposed model to prevent genetic discrimination in employment. NBCC [online], (1998).
Hayes, D. F. et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J. Natl Cancer Inst. 88, 1456–1466 (1996).
National Breast Cancer Coalition. Core Values for Breast Cancer Research. NBCC [online], (2003).
National Breast Cancer Coalition. Position statement on breast cancer quality care. NBCC [online], (2003).
Acknowledgements
The National Breast Cancer Coalition Fund (NBCCF) thanks the Strategic Consensus Conference panelists, the conference planning committee and, in particular, the consumers and scientists that worked on a pre-conference Lessons Learned Research Project: D. Berry, C. Brunswick, K. Dickersin, H. Hill, M. Mayer, H. Schiff and J. Perlmutter. The Strategic Consensus Conference was made possible thanks to generous funding provided by the Regis Foundation for Breast Cancer Research, the Virginia Clinton Kelley Fund of the NBCCF, the Doris Duke Charitable Foundation and Hurricane Voices.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
In 2006, the National Breast Cancer Coalition Fund (NBCCF) received financial support from Genomic Health, Inc. (the manufacturer of Oncotype Dx) which amounted to less that 1% of its budget. NBCCF is a collaborator in the TAILOR clinical trial, an NCI funded study that uses Oncotype Dx.
Pamela Kelin is employed by and owns stock in Genentech, Inc.
Dennis Slamon is on Genentech's speaker's bureau and sanofi–aventis speaker's bureau.
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
National Breast Cancer Coalition Fund
Strategic Consensus Conference on Biomarker Research in Breast Cancer
NCI Central Institutional Review Board Initiative
Clinical Laboratory Improvement Amendments
Association of Clinical Oncologists
National Comprehensive Cancer Network
Breast Cancer Intergroup of North America
National Surgical Adjuvant Breast and Bowel Project
NCI Cooperative Breast Cancer Tissue Resource
NCI's Cancer Diagnosis Program
Early Breast Cancer Trialists' Collaborative Group
Centers for Medicare and Medicaid Services
Agency for Healthcare Research and Quality
Rights and permissions
About this article
Cite this article
Hinestrosa, M., Dickersin, K., Klein, P. et al. Shaping the future of biomarker research in breast cancer to ensure clinical relevance. Nat Rev Cancer 7, 309–315 (2007). https://doi.org/10.1038/nrc2113
Issue Date:
DOI: https://doi.org/10.1038/nrc2113
This article is cited by
-
YAP inhibits ERα and ER+ breast cancer growth by disrupting a TEAD-ERα signaling axis
Nature Communications (2022)
-
Trace elements as tumor biomarkers and prognostic factors in breast cancer: a study through energy dispersive x-ray fluorescence
BMC Research Notes (2012)
-
Prognostic markers in cancer: the evolution of evidence from single studies to meta-analysis, and beyond
British Journal of Cancer (2009)
-
Erratum: Shaping the future of biomarker research
Nature Reviews Cancer (2007)