Key Points
-
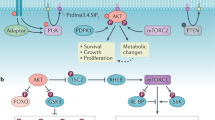
Phosphatidylinositol 3-kinases (PI3Ks) are lipid kinases that generate second messengers that govern cellular activities and properties including proliferation, survival, motility and morphology.
-
PIK3CA, the gene that encodes the catalytic subunit p110a of PI3K, is frequently mutated in human solid tumours.
-
Cancer-specific mutations are clustered in the helical and the kinase domains of p110a. The amino-acid residues E542, E545 and H1047 are prominent mutational hot spots.
-
The mutations in the p110a hot spots induce a gain of enzymatic function and confer oncogenic activity both in vitro and in vivo.
-
The oncogenicity of PI3K is the result of interactions with transcriptional and translational controls.
Abstract
There have long been indications of a role for PI3K (phosphatidylinositol 3-kinase) in cancer pathogenesis. Experimental data document a requirement for deregulation of both transcription and translation in PI3K-mediated oncogenic transformation. The recent discoveries of cancer-specific mutations in PIK3CA, the gene that encodes the catalytic subunit p110α of PI3K, have heightened the interest in the oncogenic potential of this lipid kinase and have made p110α an ideal drug target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Samuels, Y. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554 (2004). The first study to describe point mutations in the gene that encodes p110α in various solid tumours.
Bachman, K. E. et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol. Ther. 3, 772–775 (2004).
Campbell, I. G. et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 64, 7678–7681 (2004).
Broderick, D. K. et al. Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade astrocytomas, and medulloblastomas. Cancer Res. 64, 5048–5050 (2004).
Lee, J. W. et al. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene 24, 1477–1480 (2005).
Levine, D. A. et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin. Cancer Res. 11, 2875–2878 (2005).
Li, V. S. et al. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer 5, 29 (2005).
Saal, L. H. et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 65, 2554–2559 (2005).
Wang, Y., Helland, A., Holm, R., Kristensen, G. B. & Borresen-Dale, A. L. PIK3CA mutations in advanced ovarian carcinomas. Hum. Mutat. 25, 322 (2005).
Hartmann, C., Bartels, G., Gehlhaar, C., Holtkamp, N. & von Deimling, A. PIK3CA mutations in glioblastoma multiforme. Acta Neuropathol. 109, 639–642 (2005).
Vanhaesebroeck, B. & Waterfield, M. D. Signaling by distinct classes of phosphoinositide 3-kinases. Exp. Cell Res. 253, 239–254 (1999).
Cantley, L. C. The phosphoinositide 3-kinase pathway. Science 296, 1655–1657 (2002).
Okkenhaug, K. & Vanhaesebroeck, B. PI3K in lymphocyte development, differentiation and activation. Nature Rev. Immunol. 3, 317–330 (2003).
Wymann, M. P., Zvelebil, M. & Laffargue, M. Phosphoinositide 3-kinase signalling — which way to target? Trends Pharmacol. Sci. 24, 366–376 (2003).
Deane, J. A. & Fruman, D. A. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu. Rev. Immunol. 22, 563–598 (2004).
Okkenhaug, K. & Vanhaesebroeck, B. New responsibilities for the PI3K regulatory subunit p85α. Sci. STKE 65, PE1 (2001).
Rodriguez-Viciana, P. et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370, 527–532 (1994).
Rodriguez-Viciana, P., Warne, P. H., Vanhaesebroeck, B., Waterfield, M. D. & Downward, J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 15, 2442–2451 (1996).
Corvera, S. & Czech, M. P. Direct targets of phosphoinositide 3-kinase products in membrane traffic and signal transduction. Trends Cell Biol. 8, 442–446 (1998).
Balendran, A. et al. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr. Biol. 9, 393–404 (1999).
Persad, S. et al. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J. Biol. Chem. 276, 27462–27469 (2001).
Toker, A. & Newton, A. C. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. 275, 8271–8274 (2000).
Feng, J., Park, J., Cron, P., Hess, D. & Hemmings, B. A. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 279, 41189–41196 (2004).
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307, 1098–1101 (2005).
Wishart, M. J. & Dixon, J. E. PTEN and myotubularin phosphatases: from 3-phosphoinositide dephosphorylation to disease. Trends Cell Biol. 12, 579–585 (2002).
Maehama, T., Taylor, G. S. & Dixon, J. E. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu. Rev. Biochem. 70, 247–279 (2001).
Ali, I. U., Schriml, L. M. & Dean, M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J. Natl Cancer Inst. 91, 1922–1932 (1999).
Eng, C. PTEN: one gene, many syndromes. Hum. Mutat. 22, 183–198 (2003).
Leslie, N. R. & Downes, C. P. PTEN function: how normal cells control it and tumour cells lose it. Biochem. J. 382, 1–11 (2004).
Simpson, L. & Parsons, R. PTEN: life as a tumor suppressor. Exp. Cell Res. 264, 29–41 (2001).
Staal, S. P. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc. Natl Acad. Sci. USA 84, 5034–5037 (1987).
Chang, H. W. et al. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science 276, 1848–1850 (1997). Reports the isolation and identification of the v-P3k oncoprotein, a retroviral and tumorigenic homologue of p110α.
Bellacosa, A., Testa, J. R., Staal, S. P. & Tsichlis, P. N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 254, 274–277 (1991).
Aoki, M. et al. The catalytic subunit of phosphoinositide 3-kinase: requirements for oncogenicity. J. Biol. Chem. 275, 6267–6275 (2000).
Aoki, M., Batista, O., Bellacosa, A., Tsichlis, P. & Vogt, P. K. The akt kinase: molecular determinants of oncogenicity. Proc. Natl Acad. Sci. USA 95, 14950–14955 (1998).
Borlado, L. R. et al. Increased phosphoinositide 3-kinase activity induces a lymphoproliferative disorder and contributes to tumor generation in vivo. FASEB J. 14, 895–903 (2000).
Janssen, J. W., Schleithoff, L., Bartram, C. R. & Schulz, A. S. An oncogenic fusion product of the phosphatidylinositol 3-kinase p85b subunit and HUMORF8, a putative deubiquitinating enzyme. Oncogene 16, 1767–1772 (1998).
Jimenez, C. et al. Identification and characterization of a new oncogene derived from the regulatory subunit of phosphoinositide 3-kinase. EMBO J. 17, 743–753 (1998).
Jucker, M. et al. Expression of a mutated form of the p85α regulatory subunit of phosphatidylinositol 3-kinase in a Hodgkin's lymphoma-derived cell line (CO). Leukemia 16, 894–901 (2002).
Philp, A. J. et al. The phosphatidylinositol 3′-kinase p85α gene is an oncogene in human ovarian and colon tumors. Cancer Res. 61, 7426–7429 (2001).
Katoh, M. Human FOX gene family (Review). Int. J. Oncol. 25, 1495–1500 (2004).
Vogt, P. K., Jiang, H. & Aoki, M. Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle 4, 908–913 (2005).
Tran, H., Brunet, A., Griffith, E. C. & Greenberg, M. E. The many forks in FOXO's road. Sci. STKE 172, RE5 (2003).
Medema, R. H., Kops, G. J., Bos, J. L. & Burgering, B. M. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404, 782–787 (2000).
Seoane, J., Le, H. V., Shen, L., Anderson, S. A. & Massague, J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117, 211–223 (2004). Shows that FOXO1 represses p21cip1 gene expression in a complex with Smad. The oncoprotein Fox G1 binds to this complex and inhibits its repressive activity.
Schmidt, M. et al. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol. Cell. Biol. 22, 7842–7852 (2002).
Ramaswamy, S., Nakamura, N., Sansal, I., Bergeron, L. & Sellers, W. R. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell 2, 81–91 (2002).
Biggs, W. H., Meisenhelder, J., Hunter, T., Cavenee, W. K. & Arden, K. C. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl Acad. Sci. USA 96, 7421–7426 (1999).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
Kops, G. J. et al. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398, 630–634 (1999).
Takaishi, H. et al. Regulation of nuclear translocation of forkhead transcription factor AFX by protein kinase B. Proc. Natl Acad. Sci. USA 96, 11836–11841 (1999).
Tang, E. D., Nunez, G., Barr, F. G. & Guan, K. L. Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 274, 16741–16746 (1999).
Brownawell, A. M., Kops, G. J., Macara, I. G. & Burgering, B. M. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol. Cell. Biol. 21, 3534–3546 (2001).
Brunet, A. et al. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J. Cell Biol. 156, 817–828 (2002).
Rena, G., Prescott, A. R., Guo, S., Cohen, P. & Unterman, T. G. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochem. J. 354, 605–612 (2001).
Zhao, X. et al. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem. J. 378, 839–849 (2004).
Obsil, T., Ghirlando, R., Anderson, D. E., Hickman, A. B. & Dyda, F. Two 14-3-3 binding motifs are required for stable association of Forkhead transcription factor FOXO4 with 14-3-3 proteins and inhibition of DNA binding. Biochemistry 42, 15264–15272 (2003).
Perrot, V. & Rechler, M. M. The coactivator p300 directly acetylates the forkhead transcription factor Foxo1 and stimulates Foxo1-induced transcription. Mol. Endocrinol. 19, 2283–2298 (2005).
Aoki, M., Jiang, H. & Vogt, P. K. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and Akt oncoproteins. Proc. Natl Acad. Sci. USA 101, 13613–13617 (2004).
Huang, H. et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc. Natl Acad. Sci. USA 102, 1649–1654 (2005).
Plas, D. R. & Thompson, C. B. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J. Biol. Chem. 278, 12361–12366 (2003).
Matsuzaki, H., Daitoku, H., Hatta, M., Tanaka, K. & Fukamizu, A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc. Natl Acad. Sci. USA 100, 11285–11290 (2003).
Hu, M. C. et al. IκB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117, 225–237 (2004). Reports that IκB is the kinase responsible for inactivation of FOXO3a in cancer cells that lack elevated levels of phospho-AKT.
Aggarwal, B. B. Nuclear factor-κB: the enemy within. Cancer Cell 6, 203–208 (2004).
Karin, M., Cao, Y., Greten, F. R. & Li, Z. W. NF-κB in cancer: from innocent bystander to major culprit. Nature Rev. Cancer 2, 301–310 (2002).
Nakanishi, C. & Toi, M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nature Rev. Cancer 5, 297–309 (2005).
Ozes, O. N. et al. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401, 82–85 (1999).
Romashkova, J. A. & Makarov, S. S. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401, 86–90 (1999).
Kane, L. P., Mollenauer, M. N., Xu, Z., Turck, C. W. & Weiss, A. Akt-dependent phosphorylation specifically regulates Cot induction of NF-κ B-dependent transcription. Mol. Cell. Biol. 22, 5962–5974 (2002).
Madrid, L. V., Mayo, M. W., Reuther, J. Y. & Baldwin, A. S. Jr. Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-κ B through utilization of the Iκ B kinase and activation of the mitogen-activated protein kinase p38. J. Biol. Chem. 276, 18934–18940 (2001).
Sizemore, N., Leung, S. & Stark, G. R. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol. Cell. Biol. 19, 4798–4805 (1999).
Sizemore, N., Lerner, N., Dombrowski, N., Sakurai, H. & Stark, G. R. Distinct roles of the Iκ B kinase α and β subunits in liberating nuclear factor κ B (NF-κ B) from Iκ B and in phosphorylating the p65 subunit of NF-κ B. J. Biol. Chem. 277, 3863–3869 (2002).
Asano, T. et al. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-κB and c-Myc in pancreatic cancer cells. Oncogene 23, 8571–8580 (2004).
Liptay, S. et al. Mitogenic and antiapoptotic role of constitutive NF-κB/Rel activity in pancreatic cancer. Int. J. Cancer 105, 735–746 (2003).
Kim, S. et al. Down-regulation of the tumor suppressor PTEN by the tumor necrosis factor-α/nuclear factor-κB (NF-κB)-inducing kinase/NF-κB pathway is linked to a default IκB-α autoregulatory loop. J. Biol. Chem. 279, 4285–4291 (2004).
Vasudevan, K. M., Gurumurthy, S. & Rangnekar, V. M. Suppression of PTEN expression by NF-κ B prevents apoptosis. Mol. Cell. Biol. 24, 1007–1021 (2004).
Wanzel, M. et al. Akt and 14-3-3ε regulate Miz1 to control cell-cycle arrest after DNA damage. Nature Cell Biol. 7, 30–41 (2005).
Zhou, B. P. et al. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nature Cell Biol. 3, 973–982 (2001).
Mayo, L. D. & Donner, D. B. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl Acad. Sci. USA 98, 11598–11603 (2001).
Boyle, W. J. et al. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell 64, 573–584 (1991).
de Groot, R. P., Auwerx, J., Bourouis, M. & Sassone-Corsi, P. Negative regulation of Jun/AP-1: conserved function of glycogen synthase kinase 3 and the Drosophila kinase shaggy. Oncogene 8, 841–847 (1993).
Nikolakaki, E., Coffer, P. J., Hemelsoet, R., Woodgett, J. R. & Defize, L. H. Glycogen synthase kinase 3 phosphorylates Jun family members in vitro and negatively regulates their transactivating potential in intact cells. Oncogene 8, 833–840 (1993).
Gregory, M. A., Qi, Y. & Hann, S. R. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J. Biol. Chem. 278, 51606–51612 (2003).
Sears, R. et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 14, 2501–2514 (2000).
Rubinfeld, B. et al. Binding of GSK3β to the APC–β-catenin complex and regulation of complex assembly. Science 272, 1023–1026 (1996).
Cross, D. A., Alessi, D. R., Cohen, P., Andjelkovich, M. & Hemmings, B. A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 (1995).
Wei, W., Jin, J., Schlisio, S., Harper, J. W. & Kaelin, W. G. Jr. The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell 8, 25–33 (2005). Shows that GSK3 mediated phosphorylation marks c-Jun for proteasomal degradation, a mechanism that is no longer in place for oncogenic v-Jun.
Jiang, B. H. et al. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 12, 363–369 (2001).
Hamilton, G. S. & Steiner, J. P. Immunophilins: beyond immunosuppression. J. Med. Chem. 41, 5119–5143 (1998).
Vezina, C., Kudelski, A. & Sehgal, S. N. Rapamycin (AY-22, 989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. 28, 721–726 (1975).
Sabatini, D. M., Erdjument-Bromage, H., Lui, M., Tempst, P. & Snyder, S. H. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78, 35–43 (1994).
Brown, E. J. et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 369, 756–758 (1994).
Chiu, M. I., Katz, H. & Berlin, V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc. Natl Acad. Sci. USA 91, 12574–12578 (1994). References 91–93 identify mammalian TOR as a target of the FKBP12–rapamycin complex.
Chen, J., Zheng, X. F., Brown, E. J. & Schreiber, S. L. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc. Natl Acad. Sci. USA 92, 4947–4951 (1995).
Choi, J., Chen, J., Schreiber, S. L. & Clardy, J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science 273, 239–242 (1996).
Oshiro, N. et al. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells 9, 359–366 (2004).
Thomas, G., Sabatini, D. M. & Hall, N. M. (Eds.) TOR — Target of Rapamycin (Springer-Verlag, Berlin, 2004). A comprehensive compilation of literature on TOR.
Mayer, C., Zhao, J., Yuan, X. & Grummt, I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 18, 423–434 (2004). Shows that TOR regulates Pol I activity through reciprocal phosphorylation of TIF-IA.
Martin, D. E., Soulard, A. & Hall, M. N. TOR regulates ribosomal protein gene expression via PKA and the Forkhead transcription factor FHL1. Cell 119, 969–979 (2004).
Hannan, K. M. et al. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol. Cell. Biol. 23, 8862–8877 (2003).
Mahajan, P. B. Modulation of transcription of rRNA genes by rapamycin. Int. J. Immunopharmacol. 16, 711–721 (1994).
Zaragoza, D., Ghavidel, A., Heitman, J. & Schultz, M. C. Rapamycin induces the G0 program of transcriptional repression in yeast by interfering with the TOR signaling pathway. Mol. Cell. Biol. 18, 4463–4470 (1998).
Hay, N. & Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 (2004). An excellent review of the signalling network that surrounds TOR.
Montagne, J. et al. Drosophila S6 kinase: a regulator of cell size. Science 285, 2126–2129 (1999).
Radimerski, T. et al. dS6K-regulated cell growth is dPKB/dPI(3)K-independent, but requires dPDK1. Nature Cell Biol. 4, 251–255 (2002).
Fingar, D. C., Salama, S., Tsou, C., Harlow, E. & Blenis, J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 16, 1472–1487 (2002).
Pende, M. et al. S6K1−/−/S6K2−/− mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol. Cell. Biol. 24, 3112–3124 (2004).
Jefferies, H. B. et al. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J. 16, 3693–3704 (1997).
Stolovich, M. et al. Transduction of growth or mitogenic signals into translational activation of TOP mRNAs is fully reliant on the phosphatidylinositol 3-kinase-mediated pathway but requires neither S6K1 nor rpS6 phosphorylation. Mol. Cell. Biol. 22, 8101–8113 (2002).
Lin, T. A. et al. PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science 266, 653–656 (1994).
Brunn, G. J. et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277, 99–101 (1997).
Burnett, P. E., Barrow, R. K., Cohen, N. A., Snyder, S. H. & Sabatini, D. M. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl Acad. Sci. USA 95, 1432–1437 (1998).
Gingras, A. C., Kennedy, S. G., O'Leary, M. A., Sonenberg, N. & Hay, N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 12, 502–513 (1998).
Kim, D. H. et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 (2002).
Hara, K. et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177–189 (2002). References 114 and 115 report the identification of Raptor as a TOR-interacting protein necessary for the phosphorylation of TOR targets.
Nojima, H. et al. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J. Biol. Chem. 278, 15461–15464 (2003).
Schalm, S. S., Fingar, D. C., Sabatini, D. M. & Blenis, J. TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr. Biol. 13, 797–806 (2003).
Schalm, S. S. & Blenis, J. Identification of a conserved motif required for mTOR signaling. Curr. Biol. 12, 632–639 (2002).
Aoki, M., Blazek, E. & Vogt, P. K. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc. Natl Acad. Sci. USA 98, 136–141. (2001).
Penuel, E. & Martin, G. S. Transformation by v-Src: Ras–MAPK and PI3K–mTOR mediate parallel pathways. Mol. Biol. Cell 10, 1693–1703 (1999).
Neshat, M. S. et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc. Natl Acad. Sci. USA 98, 10314–10319 (2001).
Podsypanina, K. et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc. Natl Acad. Sci. USA 98, 10320–10325 (2001). References 121 and 122 demonstrate that inhibition of TOR by the rapamycin derivative CCI-779 blocks tumour growth in a PTEN -deficient setting.
Minich, W. B. & Ovchinnikov, L. P. Role of cytoplasmic mRNP proteins in translation. Biochimie 74, 477–483 (1992).
Davydova, E. K., Evdokimova, V. M., Ovchinnikov, L. P. & Hershey, J. W. Overexpression in COS cells of p50, the major core protein associated with mRNA, results in translation inhibition. Nucleic Acids Res. 25, 2911–2916 (1997).
Evdokimova, V. M. & Ovchinnikov, L. P. Translational regulation by Y-box transcription factor: involvement of the major mRNA-associated protein, p50. Int. J. Biochem. Cell Biol. 31, 139–149 (1999).
Nekrasov, M. P. et al. The mRNA-binding protein YB-1 (p50) prevents association of the eukaryotic initiation factor eIF4G with mRNA and inhibits protein synthesis at the initiation stage. J. Biol. Chem. 278, 13936–13943 (2003).
Bader, A. G., Felts, K. A., Jiang, N., Chang, H. W. & Vogt, P. K. Y box-binding protein 1 induces resistance to oncogenic transformation by the phosphatidylinositol 3-kinase pathway. Proc. Natl Acad. Sci. USA 100, 12384–12389 (2003).
Bader, A. G. & Vogt, P. K. Inhibition of protein synthesis by Y box-binding protein 1 blocks oncogenic cell transformation. Mol. Cell. Biol. 25, 2095–2106 (2005).
Preiss, T., Baron-Benhamou, J., Ansorge, W. & Hentze, M. W. Homodirectional changes in transcriptome composition and mRNA translation induced by rapamycin and heat shock. Nature Struct. Biol. 10, 1039–1047 (2003).
Grolleau, A. et al. Global and specific translational control by rapamycin in T cells uncovered by microarrays and proteomics. J. Biol. Chem. 277, 22175–22184 (2002).
Rajasekhar, V. K. et al. Oncogenic Ras and Akt signaling contribute to glioblastoma formation by differential recruitment of existing mRNAs to polysomes. Mol. Cell 12, 889–901 (2003). Reports that existing mRNAs are differentially associated with polysomes in cells that have been transformed by AKT and SRC.
Graff, J. R. & Zimmer, S. G. Translational control and metastatic progression: enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clin. Exp. Metastasis 20, 265–273 (2003).
Datta, S. R. et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91, 231–241 (1997).
del Peso, L., Gonzalez-Garcia, M., Page, C., Herrera, R. & Nunez, G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278, 687–689 (1997).
Cardone, M. H. et al. Regulation of cell death protease caspase-9 by phosphorylation. Science 282, 1318–1321 (1998).
Fujita, N., Sato, S., Katayama, K. & Tsuruo, T. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 277, 28706–28713 (2002).
Shin, I. et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27Kip1 at threonine 157 and modulation of its cellular localization. Nature Med. 8, 1145–1152 (2002).
Liang, J. et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nature Med. 8, 1153–1160 (2002).
Viglietto, G. et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27Kip1 by PKB/Akt-mediated phosphorylation in breast cancer. Nature Med. 8, 1136–1144 (2002).
Zhou, B. P. et al. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nature Cell Biol. 3, 245–252 (2001).
Kang, S., Bader, A. G. & Vogt, P. K. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc. Natl Acad. Sci. USA 102, 802–807 (2005).
Ikenoue, T. et al. Functional analysis of PIK3CA gene mutations in human colorectal cancer. Cancer Res. 65, 4562–4567 (2005). References 141 and 142 report that high-frequency mutations in p110α that are associated with human tumours are oncogenic in cultured cells.
Shayesteh, L. et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nature Genet. 21, 99–102 (1999).
Byun, D. S. et al. Frequent monoallelic deletion of PTEN and its reciprocal association with PIK3CA amplification in gastric carcinoma. Int. J. Cancer 104, 318–327 (2003).
Walker, E. H., Perisic, O., Ried, C., Stephens, L. & Williams, R. L. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature 402, 313–320 (1999).
Kang, S., Bader, A. G., Zhao, L. & Vogt, P. K. Mutated PI 3-kinases: cancer targets on a silver platter. Cell Cycle 4, 578–581 (2005).
Yu, J. et al. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110α catalytic subunit by the p85 regulatory subunit. Mol. Cell. Biol. 18, 1379–1387 (1998).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Pao, W. et al. EGF receptor gene mutations are common in lung cancers from 'never smokers' and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl Acad. Sci. USA 101, 13306–13311 (2004).
Tsao, M. S. et al. Erlotinib in lung cancer — molecular and clinical predictors of outcome. N. Engl. J. Med. 353, 133–144 (2005).
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 40, 2004–2021 (2001).
Manetsch, R. et al. In situ click chemistry: enzyme inhibitors made to their own specifications. J. Am. Chem. Soc. 126, 12809–12818 (2004).
Mocharla, V. P. et al. In situ click chemistry: enzyme-generated inhibitors of carbonic anhydrase II. Angew. Chem. Int. Ed. Engl. 44, 116–120 (2004).
Inoki, K., Li, Y., Xu, T. & Guan, K. L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17, 1829–1834 (2003).
Tee, A. R., Manning, B. D., Roux, P. P., Cantley, L. C. & Blenis, J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 13, 1259–1268 (2003).
Saucedo, L. J. et al. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nature Cell Biol. 5, 566–571 (2003).
Garami, A. et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol. Cell 11, 1457–1466 (2003).
Zhang, Y. et al. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nature Cell Biol. 5, 578–581 (2003).
Inoki, K., Li, Y., Zhu, T., Wu, J. & Guan, K. L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nature Cell Biol. 4, 648–657 (2002).
Long, X., Lin, Y., Ortiz-Vega, S., Yonezawa, K. & Avruch, J. Rheb binds and regulates the mTOR kinase. Curr. Biol. 15, 702–713 (2005).
Treins, C., Giorgetti-Peraldi, S., Murdaca, J., Semenza, G. L. & Van Obberghen, E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J. Biol. Chem. 277, 27975–27981 (2002).
Hudson, C. C. et al. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22, 7004–7014 (2002).
Massion, P. P. et al. Early involvement of the phosphatidylinositol 3-kinase/Akt pathway in lung cancer progression. Am. J. Respir. Crit. Care Med. 170, 1088–1094 (2004).
Pedrero, J. M. et al. Frequent genetic and biochemical alterations of the PI 3-K/AKT/PTEN pathway in head and neck squamous cell carcinoma. Int. J. Cancer 114, 242–248 (2005).
Ma, Y. Y. et al. PIK3CA as an oncogene in cervical cancer. Oncogene 19, 2739–2744 (2000).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nature Rev. Cancer 2, 489–501 (2002).
Cheng, J. Q. et al. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc. Natl Acad. Sci. USA 93, 3636–3641 (1996).
Balsara, B. R. et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis 25, 2053–2059 (2004).
Min, Y. H. et al. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia 17, 995–997 (2003).
Acknowledgements
The work of the authors is supported by grants from the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
National Cancer Institute
Swiss-Prot
FURTHER INFORMATION
Atlas of Genetics and Cytogenetics in Oncology and Haematology (PIK3CA)
Glossary
- SH2 DOMAIN
-
A protein domain, homologous to an equivalent domain in the SRC protein, that facilitates protein–protein interactions by binding to tyrosine-phosphorylated protein sequences.
- PLECKSTRIN HOMOLOGY DOMAIN
-
A protein domain that was originally identified in the protein pleckstrin that forms a phospholipid-binding pocket and in particular binds to PIP3.
- MACROLIDE
-
A group of antibiotics that are produced by various strains of Streptomyces and have a complex macrocyclic structure.
- POL I- AND POL III-DEPENDENT TRANSCRIPTION
-
Class I RNA polymerases synthesize all ribosomal RNAs except the 5S ribosomal RNA; class III RNA polymerases transcribe transfer RNA genes and the gene that encodes the 5S ribosomal RNA.
- mRNA CAP STRUCTURE
-
The 5′ end of virtually all mRNAs is protected by a 'cap' with the chemical structure m7GpppN (where m7G represents 7-methylguanylate, p represents a phosphate group and N represents any base).
Rights and permissions
About this article
Cite this article
Bader, A., Kang, S., Zhao, L. et al. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer 5, 921–929 (2005). https://doi.org/10.1038/nrc1753
Issue Date:
DOI: https://doi.org/10.1038/nrc1753
This article is cited by
-
Targeting PI3K/Akt in Cerebral Ischemia Reperfusion Injury Alleviation: From Signaling Networks to Targeted Therapy
Molecular Neurobiology (2024)
-
Combined treatment with ruxolitinib and MK-2206 inhibits the JAK2/STAT5 and PI3K/AKT pathways via apoptosis in MDA-MB-231 breast cancer cell line
Molecular Biology Reports (2023)
-
Pharmacokinetics and Pharmacodynamic of Alpelisib
Clinical Pharmacokinetics (2023)
-
Function of Long Noncoding RNAs in Glioma Progression and Treatment Based on the Wnt/β-Catenin and PI3K/AKT Signaling Pathways
Cellular and Molecular Neurobiology (2023)
-
TPI1 activates the PI3K/AKT/mTOR signaling pathway to induce breast cancer progression by stabilizing CDCA5
Journal of Translational Medicine (2022)