Key Points
-
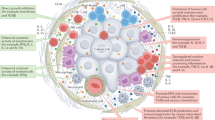
Immune-cell infiltrates constitute a prominent component of the host response to cancer in some cases, but their functional significance remains incompletely understood.
-
Cancer cells express antigens that can be recognized by both the innate and adaptive immune systems.
-
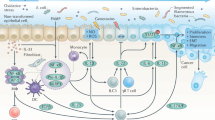
Host-derived cytokines can suppress tumour formation by controlling infection, inflammation and immunity.
-
Tumour cells can exploit host-derived cytokines to promote growth, increase resistance to apoptosis and foster dissemination.
-
The systemic administration of cytokines can elicit antitumour effects, but the toxicities that are associated with this treatment often resemble a state of severe infection and can therefore be limiting.
-
Tumour cells can be genetically modified to express particular cytokines that stimulate the host immune response, thereby acquiring the capacity to function as cancer vaccines.
-
Antibody blockade of the CTLA-4 inhibitory receptor on T cells is a promising strategy to increase the potency of cancer vaccines, albeit with a risk of compromising tolerance to self-antigens.
Abstract
The mixture of cytokines that is produced in the tumour microenvironment has an important role in cancer pathogenesis. Cytokines that are released in response to infection, inflammation and immunity can function to inhibit tumour development and progression. Alternatively, cancer cells can respond to host-derived cytokines that promote growth, attenuate apoptosis and facilitate invasion and metastasis. A more detailed understanding of cytokine–tumour-cell interactions provides new opportunities for improving cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Liotta, L. A. & Kohn, E. C. The microenvironment of the tumour–host interface. Nature 411, 375–379 (2001).
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med. 1, 27–31 (1995).
Clark, W. et al. Model predicting survival in stage I melanoma based on tumor progression. J. Natl. Cancer Inst. 81, 1893–1904 (1989). This study was the first to demonstrate that intratumoral T-cell infiltrates are correlated with improved clinical outcomes in human melanoma.
Clemente, C. et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77, 1303–1310 (1996).
Naito, Y. et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 58, 3491–3494 (1998).
Nakano, O. et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 61, 5132–5136 (2001).
Zhang, L. et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Eng. J. Med. 348, 203–213 (2003).
Dunn, G., Bruce, A., Ikeda, H., Old, L. & Schreiber, R. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunol. 3, 991–998 (2002).
Ames, B. N., Gold, L. S. & Willett, W. C. The causes and prevention of cancer. Proc. Natl Acad. Sci. USA 92, 5258–5265 (1995).
Coussens, L. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Ochsenbein, A. F. et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature 411, 1058–1064 (2001).
Dranoff, G. & Mulligan, R. C. Gene transfer as cancer therapy. Adv. Immunol. 58, 417–454 (1995).
Paul, W. E. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell 57, 521–524 (1989).
Janeway, C. A. Jr. How the immune system works to protect the host from infection: a personal view. Proc. Natl Acad. Sci. USA 98, 7461–7468 (2001).
Zinkernagel, R. M. On natural and artificial vaccinations. Annu. Rev. Immunol. 21, 515–546 (2003).
Diefenbach, A. & Raulet, D. The innate immune response to tumors and its role in the induction of T-cell immunity. Immunol. Rev. 188, 9–21 (2002).
Karre, K. NK cells, MHC class I molecules and the missing self. Scan. J. Immunol. 55, 221–228 (2002).
Albert, M. et al. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188, 1359–1368 (1998).
Basu, S., Binder, R. J., Ramalingam, T. & Srivastava, P. K. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14, 303–313 (2001).
Sharpe, A. H. & Freeman, G. J. The B7-CD28 superfamily. Nature Rev. Immunol. 2, 116–126 (2002).
Banchereau, J. & Steinman, R. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Boon, T. & van der Bruggen, P. Human tumor antigens recognized by T lymphocytes. J. Exp. Med. 183, 725–729 (1996).
Old, L. & Chen, Y. -T. New paths in human cancer serology. J. Exp. Med. 187, 1163–1167 (1998).
Sahin, U. et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc. Natl Acad. Sci. USA 92, 11810–11813 (1995).
Marincola, F. M., Jaffee, E. M., Hicklin, D. J. & Ferrone, S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 74, 181–273 (2000).
Kaplan, D. et al. Demonstration of an interferon γ-dependent tumor surveillance system in immunocompetent mice. Proc. Natl Acad. Sci. USA 95, 7556–7561 (1998).
Street, S. E., Cretney, E. & Smyth, M. J. Perforin and interferon-γ activities independently control tumor initiation, growth, and metastasis. Blood 97, 192–197 (2001).
Qin, Z., Kim, H. J., Hemme, J. & Blankenstein, T. Inhibition of methylcholanthrene-induced carcinogenesis by an interferon γ receptor-dependent foreign body reaction. J. Exp. Med. 195, 1479–1490 (2002).
Bach, E. A., Aguet, M. & Schreiber, R. D. The IFNγ receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 15, 563–591 (1997).
Smyth, M. et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 191, 661–668 (2000).
Shankaran, V. et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001). This study delineates an increased incidence of spontaneous carcinomas in mice deficient in Stat1 and Rag2.
Girardi, M. et al. Regulation of cutaneous malignancy by γδ T cells. Science 294, 605–609 (2001).
Girardi, M. et al. Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J. Exp. Med. 195, 855–867 (2002).
Gao, Y. et al. γδ T cells provide an early source of interferon γ in tumor immunity. J. Exp. Med. 198, 433–442 (2003).
van den Broek, M. et al. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 184, 1781–1790 (1996).
Takeda, K. et al. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J. Exp. Med. 195, 161–169 (2002).
Smyth, M. et al. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 192, 755–760 (2000).
Street, S. E., Trapani, J. A., MacGregor, D. & Smyth, M. J. Suppression of lymphoma and epithelial malignancies effected by interferon γ. J. Exp. Med. 196, 129–134 (2002).
Davidson, W., Giese, T. & Fredrickson, T. Spontaneous development of plasmacytoid tumors in mice with defective fas–fas ligand interactions. J. Exp. Med. 187, 1825–1838 (1998).
Siegel, R. M., Chan, F. K., Chun, H. J. & Lenardo, M. J. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nature Immunol. 1, 469–474 (2000).
Enzler, T. et al. Deficiencies of GM-CSF and interferon-γ link inflammation and cancer. J. Exp. Med. 197, 1213–1219 (2003). This study delineates a crucial role for microbial agents in the spontaneous development of lymphomas and solid tumors in Gm-csf/Ifn-γ -deficient mice.
Kado, S. et al. Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor β chain and p53 double-knockout mice. Cancer Res. 61, 2395–2398 (2001).
Engle, S. J. et al. Elimination of colon cancer in germ-free transforming growth factor β1-deficient mice. Cancer Res. 62, 6362–6366 (2002).
Penn, I. Depressed immunity and the development of cancer. Cancer Detect. Prev. 18, 241–252 (1994).
Kuper, H., Adami, H. O. & Trichopoulos, D. Infections as a major preventable cause of human cancer. J. Intern. Med. 248, 171–183 (2000).
Pisani, P., Parkin, D. M., Munoz, N. & Ferlay, J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol. Biomarkers Prev. 6, 387–400 (1997).
El-Omar, E. M. et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404, 398–402 (2000).
Nakamoto, Y., Guidotti, L. G., Kuhlen, C. V., Fowler, P. & Chisari, F. V. Immune pathogenesis of hepatocellular carcinoma. J. Exp. Med. 188, 341–350 (1998).
Nakamoto, Y. et al. Prevention of hepatocellular carcinoma development associated with chronic hepatitis by anti-fas ligand antibody therapy. J. Exp. Med. 196, 1105–1111 (2002).
Daniel, D. et al. Immune enhancement of skin carcinogenesis by CD4+ T cells. J. Exp. Med. 197, 1017–1028 (2003).
Fehniger, T. et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J. Exp. Med. 193, 219–231 (2001).
Hudson, J. et al. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 190, 1375–1382 (1999).
Moore, R. J. et al. Mice deficient in tumor necrosis factor-α are resistant to skin carcinogenesis. Nature Med. 5, 828–831 (1999).
Hilbert, D. M., Kopf, M., Mock, B. A., Kohler, G. & Rudikoff, S. Interleukin 6 is essential for in vivo development of B lineage neoplasms. J. Exp. Med. 182, 243–248 (1995).
Lattanzio, G. et al. Defective development of pristane-oil-induced plasmacytomas in interleukin-6-deficient BALB/c mice. Am. J. Pathol. 151, 689–696 (1997).
Lin, E., Nguyen, A., Russell, R. & Pollard, J. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 193, 727–739 (2001).
Voronov, E. et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl Acad. Sci. USA 100, 2645–2650 (2003).
Coussens, L. M., Tinkle, C. L., Hanahan, D. & Werb, Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103, 481–490 (2000). This study demonstrates that immune cells can promote tumour formation and progression.
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001).
Steinbach, G. et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Eng. J. Med. 342, 1946–1952 (2000).
Jacoby, R. F., Seibert, K., Cole, C. E., Kelloff, G. & Lubet, R. A. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 60, 5040–5044 (2000).
Nauts, H., Fowler, G. & Bogatko, F. A review of the influence of bacterial infection and of bacterial products (Coley's toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley's mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study. Acta Med. Scand. 144, S1–S103 (1953). This report documents durable clinical remissions in response to mixed bacterial toxins.
Interferon α versus chemotherapy for chronic myeloid leukemia: a meta-analysis of seven randomized trials: Chronic Myeloid Leukemia Trialists' Collaborative Group. J. Natl. Cancer Inst. 89, 1616–1620 (1997).
Kirkwood, J. et al. Interferon α-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J. Clin. Oncol. 14, 7–17 (1996).
Kirkwood, J. M. et al. High-dose interferon α-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J. Clin. Oncol. 19, 2370–2380 (2001).
Fyfe, G. et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 13, 688–696 (1995).
Rosenberg, S. A. et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J. Natl. Cancer Inst. 85, 622–632 (1993).
Soiffer, R. J. et al. Expansion and manipulation of natural killer cells in patients with metastatic cancer by low-dose continuous infusion and intermittent bolus administration of interleukin 2. Clin. Cancer Res. 2, 493–499 (1996).
Furtado, G. C., Curotto de Lafaille, M. A., Kutchukhidze, N. & Lafaille, J. J. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J. Exp. Med. 196, 851–857 (2002).
Lienard, D., Ewalenko, P., Delmotte, J. J., Renard, N. & Lejeune, F. J. High-dose recombinant tumor necrosis factor α in combination with interferon γ and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J. Clin. Oncol. 10, 52–60 (1992).
Atkins, M. B. et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. 3, 409–417 (1997).
Spitler, L. E. et al. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte–macrophage colony-stimulating factor. J. Clin. Oncol. 18, 1614–1621 (2000).
Rini, B. I., Weinberg, V., Bok, R. & Small, E. J. Prostate-specific antigen kinetics as a measure of the biologic effect of granulocyte–macrophage colony-stimulating factor in patients with serologic progression of prostate cancer. J. Clin. Oncol. 21, 99–105 (2003).
Anderson, P. M. et al. Aerosol granulocyte macrophage–colony stimulating factor: a low toxicity, lung-specific biological therapy in patients with lung metastases. Clin. Cancer Res. 5, 2316–2323 (1999).
Lieschke, G. J. & Burgess, A. W. Granulocyte colony-stimulating factor and granulocyte–macrophage colony-stimulating factor. N. Eng. J. Med. 327, 28–35, 99–106 (1992).
Demetri, G. D., Kris, M., Wade, J., Degos, L. & Cella, D. Quality-of-life benefit in chemotherapy patients treated with epoetin α is independent of disease response or tumor type: results from a prospective community oncology study. Procrit Study Group. J. Clin. Oncol. 16, 3412–3425 (1998).
Forni, G. et al. Helper strategy in tumor immunology: expansion of helper lymphocytes and utilization of helper lymphokines for experimental and clinical immunotherapy. Cancer Metast. Rev. 7, 289–309 (1988).
Dranoff, G. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte–macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA 90, 3539–3543 (1993).
Cavallo, F. et al. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J. Natl. Cancer Inst. 89, 1049–1058 (1997).
Boggio, K. et al. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinoma in two lines of her-2/neu transgenic mice. J. Exp. Med. 188, 589–596 (1998).
Mach, N. et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte–macrophage colony-stimulating factor or flt3-ligand. Cancer Res. 60, 3239–3246 (2000).
Gillessen, S. et al. CD1d-restricted T cells regulate dendritic cell function and antitumor immunity in a granulocyte–macrophage colony-stimulating factor-dependent fashion. Proc. Natl Acad. Sci. USA 100, 8874–8879 (2003).
Hung, K. et al. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 188, 2357–2368 (1998).
Reilly, R. et al. The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res. 61, 880–883 (2001).
Curcio, C. et al. Nonredundant roles of antibody, cytokines, and perforin in the eradication of established Her-2/neu carcinomas. J. Clin. Invest. 111, 1161–1170 (2003).
Mach, N. & Dranoff, G. Cytokine-secreting tumor cell vaccines. Curr. Opin. Immunol. 12, 571–575 (2000).
Soiffer, R. et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete human granulocyte–macrophage colony stimulating factor generates potent anti-tumor immunity in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 95, 13141–13146 (1998).
Schmollinger, J. C. et al. Melanoma inhibitor of apoptosis protein (ML-IAP) is a target for immune-mediated tumor destruction. Proc. Natl Acad. Sci. USA 100, 3398–3403 (2003).
Hodi, F. S. et al. ATP6S1 elicits potent humoral responses associated with immune mediated tumor destruction. Proc. Natl Acad. Sci. USA 99, 6919–6924 (2002).
Mollick, J. A., Hodi, F. S., Soiffer, R. J., Nadler, L. M. & Dranoff, G. MUC1-like tandem repeat proteins are broadly immunogenic in cancer patients. Cancer Immun. 3, 3–20 (2003).
Simons, J. W. et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte–macrophage colony-stimulating factor gene transfer. Cancer Res. 57, 1537–1546 (1997).
Simons, J. et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 59, 5160–5168 (1999).
Zehnter, S. et al. Tumor metastasis biopsy as a surrogate marker of response to melanoma immunotherapy. Pathology 31, 116–122 (1999).
Shenk, T. in Fields Virology (eds Fields, B. N., Knipe, D. M. & Howley, P. M.) 2111–2148 (Lippincott–Raven Publishers, Philadelphia, 1996).
Salgia, R. et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte–macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J. Clin. Oncol. 21, 624–630 (2003).
Borrello, I., Sotomayor, E., Cooke, S. & Levitsky, H. A universal granulocyte–macrophage colony-stimulating factor-producing bystander cell line for use in the formulation of autologous tumor cell-based vaccines. Hum. Gene Ther. 10, 1983–1991 (1999).
Jaffee, E. et al. Novel allogeneic granulocyte–macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J. Clin. Oncol. 19, 145–156 (2001).
Chambers, C. A., Kuhns, M. S., Egen, J. G. & Allison, J. P. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 19, 565–594 (2001).
Doyle, A. M. et al. Induction of cytotoxic T lymphocyte antigen 4 (CTLA-4) restricts clonal expansion of helper T cells. J. Exp. Med. 194, 893–902 (2001).
Salomon, B. & Bluestone, J. A. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19, 225–252 (2001).
Shevach, E. M., McHugh, R. S., Piccirillo, C. A. & Thornton, A. M. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol. Rev. 182, 58–67 (2001).
van Elsas, A., Hurwitz, A. & Allison, J. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190, 355–366 (1999).
Sutmuller, R. P. et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J. Exp. Med. 194, 823–832 (2001).
Waterhouse, P. et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 (1995).
Tivol, E. A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995).
Phan, G. Q. et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 100, 8372–8377 (2003).
Hodi, F. S. et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl Acad. Sci. USA 100, 4712–4717 (2003). This study, together with reference 107, indicates that CTLA-4 antibody blockade can be combined with cancer vaccines to increase tumour immunity in patients, albeit with a risk of compromising tolerance to self-antigens.
Herr, H. et al. Intravesical bacille Calmette-Guérin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. J. Clin. Oncol. 13, 1404–1408 (1995). This study demonstrates that the therapeutic administration of a mycobacteria reduces morbidity and mortality of bladder carcinoma.
Driggers, P. H., Elenbaas, B. A., An, J. B., Lee, I. J. & Ozato, K. Two upstream elements activate transcription of a major histocompatibility complex class I gene in vitro. Nuc. Acids Res. 20, 2533–2540 (1992).
Holtschke, T. et al. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 87, 307–317 (1996).
Hao, S. X. & Ren, R. Expression of interferon consensus sequence binding protein (ICSBP) is downregulated in Bcr–Abl-induced murine chronic myelogenous leukemia-like disease, and forced coexpression of ICSBP inhibits Bcr-Abl-induced myeloproliferative disorder. Mol. Cell Biol. 20, 1149–1161 (2000).
Schiavoni, G. et al. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8α(+) dendritic cells. J. Exp. Med. 196, 1415–1425 (2002).
Schmidt, M. et al. Lack of interferon consensus sequence binding protein (ICSBP) transcripts in human myeloid leukemias. Blood 91, 22–29 (1998).
Takaoka, A. et al. Integration of interferon-α/β signalling to p53 responses in tumour suppression and antiviral defence. Nature 424, 516–523 (2003).
Molldrem, J. et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nature Med. 6, 1018–1023 (2000).
Molldrem, J. J. et al. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J. Clin. Invest. 111, 639–647 (2003).
Fearon, E. R. et al. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell 60, 397–403 (1990).
Gansbacher, B. et al. Interleukin-2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J. Exp. Med. 172, 1217–1224 (1990).
Pulaski, B. A. et al. Interleukin 3 enhances cytotoxic T lymphocyte development and class I major histocompatibility complex re-presentation of exogenous antigen by tumor-infiltrating antigen-presenting cells. Proc. Natl Acad. Sci. USA 93, 3669–3674 (1996).
Tepper, R. I., Coffman, R. L. & Leder, P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 257, 548–551 (1992).
Golumbek, P. T. et al. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science 254, 713–716 (1991).
Nishimoto, N. et al. Improvement in Castleman's disease by humanized anti-interleukin-6 receptor antibody therapy. Blood 95, 56–61 (2000).
Hock, H., Dorsch, M., Diamanstein, T. & Blankenstein, T. Interleukin-7 induces CD4+ T cell-dependent tumor rejection. J. Exp. Med. 174, 1291–1298 (1991).
Qin, Z., Noffz, G., Mohaupt, M. & Blankenstein, T. Interleukin-10 prevents dendritic cell accumulation and vaccination with granulocyte-macrophage colony-stimulating factor gene-modified tumor cells. J. Immunol. 159, 770–776 (1997).
Brunda, M. J. et al. Antitumor and antimetastatic activity of interleukin-12 against murine tumors. J. Exp. Med. 178, 1223–1230 (1993).
Terabe, M. et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R–STAT6 pathway. Nature Immunol. 1, 515–520 (2000).
Hazama, S. et al. Tumour cells engineered to secrete interleukin-15 augment anti-tumour immune responses in vivo. Br. J. Cancer 80, 1420–1426 (1999).
Micallef, M. J., Tanimoto, T., Kohno, K., Ikeda, M. & Kurimoto, M. Interleukin 18 induces the sequential activation of natural killer cells and cytotoxic T lymphocytes to protect syngeneic mice from transplantation with Meth A sarcoma. Cancer Res. 57, 4557–4563 (1997).
Jakubowski, A. A. et al. Phase I study of continuous-infusion recombinant macrophage colony-stimulating factor in patients with metastatic melanoma. Clin. Cancer Res. 2, 295–302 (1996).
Biron, C. A. Interferons α and β as immune regulators: a new look. Immunity 14, 661–664 (2001).
Tracey, K. J. & Cerami, A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu. Rev. Med. 45, 491–503 (1994).
Walczak, H. et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nature Med. 5, 157–163 (1999).
Lynch, D. et al. Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nature Med. 3, 625–631 (1997).
Rousseau, R. F. et al. Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood 101, 1718–1726 (2003).
Gorelik, L. & Flavell, R. A. Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nature Med. 7, 1118–1122 (2001).
Acknowledgements
Supported by National Institutes of Health grants and the Leukemia and Lymphoma Society.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares clinical trial support from Cell Genesys and Medarex.
Related links
Related links
DATABASES
Cancer.gov
LocusLink
Glossary
- AUTOCRINE
-
A form of bioregulation in which a secretory factor affects only the cell from which it was secreted.
- PARACRINE
-
A form of bioregulation in which a cytokine that is released from one cell triggers a specific response from another cell in the microenvironment.
- COMPLEMENT PROTEINS
-
A set of plasma proteins, activated as a proteolytic cascade, that functions to coat the surface of microbes, thereby stimulating their lysis or phagocytosis.
- CD4+ T CELLS
-
A subset of lymphocytes that develops in the thymus and recognizes peptides that are presented by major histocompatibility class II antigens. These cells produce a wide range of cytokines that enhance B-cell antibody production and CD8+ T-cell and macrophage cytotoxic function.
- CD8+ T CELLS
-
A subset of lymphocytes that develops in the thymus and recognizes peptides presented by major histocompatibility class I antigens. These cells kill targets primarily through the perforin–granzyme pathway and death ligands such as FAS and tumour-necrosis factor.
- MAJOR HISTOCOMPATIBILITY COMPLEX
-
(MHC). Locus of genes that encode a set of highly polymorphic membrane glycoproteins that present processed peptides to T-cell receptors.
- γδ T CELL
-
A minor population of thymic-derived lymphocytes that express a heterodimeric γδ T-cell receptor. These lymphocytes can respond to glycolipid antigens and function at the interface of innate and adaptive immunity.
- CROSS PRIMING
-
Initiation of a CD8+ T-cell response against an antigen that is not present within antigen-presenting cells. The antigen must be taken up by antigen-presenting cells and then re-routed to the major histocompatibility class I presentation pathway.
- IMMUNOGENICITY
-
The ability of an antigen or vaccine to stimulate an immune response.
- CHEMOKINES
-
A family of chemotactic proteins that are divided into C, CC, CXC and CX3C chemokines, depending on the number and spacing of conserved cysteine residues in the amino-terminal part of the protein. Chemokines are involved in inflammatory-cell recruitment and act through G-protein-coupled receptors.
- SYNGENEIC
-
Genetically identical, for example, a fully inbred strain of mouse.
- TH1 RESPONSE
-
A T-helper-1 immune response is mediated by pro-inflammatory cytokines that are expressed by CD4+ T cells, such as interferon-γ and tumour necrosis factor-β. It promotes cellular immune responses against intracellular infections and malignancy.
- TH2 RESPONSE
-
A T-helper-2 immune response involves production, by CD4+ T cells, of cytokines such as interleukin-4, which stimulate antibody production. TH2 cytokines promote secretory immune responses of mucosal surfaces to extracellular pathogens and allergic reactions.
- ALLOGENEIC
-
Cells that are derived from the same species, but that differ in the expression of major histocompatibility complex alleles and other genetic polymorphisms.
- OEDEMA
-
The accumulation of fluid in the intercellular spaces in tissues, reflecting an increase in vascular permeability or pressure.
- ENTEROCOLITIS
-
Inflammatory disease of the small and large intestine, resulting in significant diarrhoea.
- HYPOPHYSITIS
-
Inflammation of the pituitary gland that results in significant disturbances in endocrine function.
Rights and permissions
About this article
Cite this article
Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer 4, 11–22 (2004). https://doi.org/10.1038/nrc1252
Issue Date:
DOI: https://doi.org/10.1038/nrc1252
This article is cited by
-
Time-series blood cytokine profiles correlate with treatment responses in triple-negative breast cancer patients
British Journal of Cancer (2024)
-
Mechanism-centric regulatory network identifies NME2 and MYC programs as markers of Enzalutamide resistance in CRPC
Nature Communications (2024)
-
Upconversion nanoparticles and their potential in the realm of biomedical sciences and theranostics
Journal of Nanoparticle Research (2024)
-
Oncolytic virus-based suicide gene therapy for cancer treatment: a perspective of the clinical trials conducted at Henry Ford Health
Translational Medicine Communications (2023)
-
Reprogramming the tumor microenvironment with biotechnology
Biomaterials Research (2023)